The purpose of this analysis was to assess the incidence, predictors and prognostic impact of acute heart failure (AHF) after transcatheter aortic valve implantation (TAVI) using a self-expanding prosthesis.
MethodsFrom November 2008 to June 2017, all consecutive patients undergoing TAVI in our center were prospectively included in our TAVI registry. The predictive effect of AHF on all-cause mortality following the TAVI procedure was analyzed using Cox regression models.
ResultsA total of 399 patients underwent TAVI with a mean age of 82.4 ± 5.8 years, of which 213 (53.4%) were women. During follow-up (27.0 ± 24.1 months), 29.8% (n = 119) were admitted due to AHF, which represents a cumulative incidence function of 13.2% (95%CI, 11.1%-15.8%). At the end of follow-up, 150 patients (37.59%) had died. Those who developed AHF showed a significantly higher mortality rate (52.1% vs 31.4%; HR, 1.84; 95%; CI, 1.14-2.97; P = .012). Independent predictors of AHF after TAVI were a past history of heart failure (P = .019) and high Society of Thoracic Surgeons score (P = .004). We found that nutritional risk index and chronic obstructive pulmonary disease were strongly correlated with outcomes in the AHF group.
ConclusionsTAVI was associated with a high incidence of clinical AHF. Those who developed AHF had higher mortality. Pre-TAVI AHF and high Society of Thoracic Surgeons score were the only independent predictors of AHF in our cohort. A low nutritional risk index and chronic obstructive pulmonary disease were independent markers of mortality in the AHF group.
Keywords
Degenerative aortic stenosis (AS) has become the most prevalent valvular heart disease in developed countries with an increasing incidence due to progressive population aging.1,2 Transcatheter aortic valve implantation (TAVI) has been shown to reduce mortality compared with conservative medical treatment in patients with severe AS. It is also a solid option for treating patients with high or prohibitive surgical risk, as an alternative to conventional surgical aortic valve replacement.3,4 In addition, 2 recent studies, PARTNER 25 and SURTAVI,6 demonstrated the noninferiority of TAVI vs conventional surgery in patients at intermediate risk, with superior results when the femoral approach was used.7
Patients selected for TAVI commonly have important comorbidities that determine the in-hospital course and can result in a higher number of postprocedure readmissions. The number of readmissions has decreased over the past few years due to increased operator expertise and fewer complications with newer devices and delivery systems. Acute heart failure (AHF) is one of the most prevalent causes of readmission in this group of patients, as reported in several series and registries.8,9
Risk scores have become an important tool for predicting procedural and periprocedural outcome following TAVI. The logistic EuroSCORE (European System for Cardiac Operative Risk Evaluation) was shown to overestimate the periprocedural risk in TAVI, especially in high-risk patients and was abandoned. The EuroSCORE II and the Society of Thoracic Surgeons (STS) score were proved to be more accurate for TAVI patients and are therefore currently used to estimate risk of death in patients undergoing TAVI.10,11
In this study, we analyzed the incidence of rehospitalization after TAVI in patients with main diagnosis of AHF and focused on the prognostic impact for our cohort. In addition, we searched for predictors of readmission due to AHF, and analyzed the factors that could modify the prognosis in this subgroup. This would help us to identify a profile of patients at high risk of complications. Intensification of medical treatment and closer follow-up may allow us to avoid readmissions, improving the quality of life of these patients.
METHODSPopulationThis was an observational, single center, prospective study. We included all patients who underwent TAVI in our university hospital from November 2008 to June 2017 (n = 399) were included. All patients were selected for transcatheter replacement according to the clinical practice guideline recommendations available at the time; only patients with a life expectancy of more than 1 year and severe symptomatic aortic stenosis were included. The indication for TAVI was made according to the guidelines available at the time of inclusion. All patients had been previously discussed by a Heart Team consisting of clinical cardiologists, interventional cardiologists, and cardiac surgeons. All patients voluntarily signed consent forms before the procedure.
The major factors that contributed to the decision to perform the transcatheter procedure vs surgical aortic valve replacement were high or unacceptable surgical risk, frailty associated with older age, and technical contraindications for surgery (most frequently the presence of porcelain aorta).
ProcedureIn most patients, TAVI was performed under local anesthesia and conscious light sedation. General anesthesia was used in 8% (n = 30) of procedures, when the nonfemoral arterial approach was preferred. The femoral approach was used in most procedures. When this was not feasible, axillar artery was the selected approach. We used the standard technique as described in the literature.12
A Medtronic biological prosthesis were implanted in most patients: CoreValve, CoreValve Evolut R or CoreValve Evolut Pro (Medtronic Inc., Minneapolis, MN, USA), depending on the availability at the time of the procedure. In a small percentage of patients, ACURATE-Neo (Symetis S.A., Ecublens, Switzerland) devices were used.
Before the intervention, 2-dimensional echocardiography and diagnostic coronary angiography were performed in all patients. As part of our routine protocol, computed tomography was used to determine aortic root anatomy and adequacy of vascular accesses. Any complication during the procedure was registered according to Valve Academic Research Consortium-2 (VARC-2) consensus document.13
This study was performed in accordance with the principles of the Helsinki Declaration.
Follow-upAll data related to the event were registered in the patients’ electronic medical records. In our TAVI Registry, follow-up was performed using previous registries by trained cardiologists. Our protocol includes telephone calls and review of the electronic medical records. All medical interventions, hospital admissions and pharmacological treatments were reviewed. Vital status was determined by telephone calls in the absence of medical records. No patient was lost to follow-up.
Following the established protocol at least 1 follow-up echocardiogram was performed in all patients after discharge and another one 3 months later. Subsequently an annual echocardiogram was performed.
Study variablesAHF was defined following the available practice guidelines at the time of recruitment,14 based on clinical and radiological data. The nutritional risk index (NRI) was calculated as 1.519 × serum albumin (g/L) + 41.7 × (actual body weight [kg]/ideal body weight [kg]), using the modified formula for the elderly by Bouillanne et al.15 Ideal body weight was determined using the Lorentz formula:15 height (cm) −100 −([height (cm) − 150]/4) for men, or height (cm) −100 −([height (cm) −150]/2.5) for women. If the ratio of measured body weight (kg) to ideal body weight (kg) was ≥ 1, the assigned value was 1, as previously described.15,16 The NRI was calculated using the body weight measured on the day of the TAVI procedure, and the albumin value was obtained from the blood sample performed the day before the procedure. Based on NRI values, we classified the patients into 4 groups: no nutritional risk (NRI > 100), mild nutritional risk (97.5 ≤ NRI < 100), moderate nutritional risk (83.5 ≤ NRI < 97.4), and severe nutritional risk (NRI < 83.5). To simplify the model, we obtained 2 risk categories after the combination of the following: no and mild nutritional risk, and moderate and severe nutritional risk.
The presence and severity of paravalvular aortic regurgitation was assessed using transthoracic echocardiography. Transesophageal echocardiography was performed in those patients with a suboptimal acoustic window.
Statistical analysisThe statistical analysis was performed with SPSS version 22.0 and Stata version 13. Baseline characteristics according to the development of post-TAVI heart failure (HF) during follow-up are described using number and percentage for categorical data and mean ± standard deviation for continuous data, respectively. Differences in characteristics were assessed by using chi-square tests and 2-sample Student t tests.
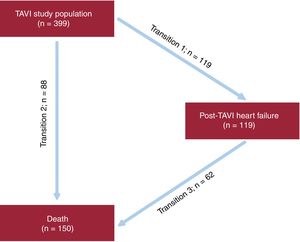
The association between post-TAVI HF and mortality was evaluated by Cox proportional hazards regression analyses with “post-TAVI HF” as a time-varying covariate. Results were graphically shown with Kaplan-Meier curves. Because HF hospitalization and death are semicompeting risks in which death precludes a subsequent HF hospitalization but death can still occur after a HF hospitalization, an illness-death, acyclic, multistate model was used.17 In this model, all participants were in the initial state of “discharge after TAVI” and were at risk of a HF hospitalization (transition 1) or death without a preceding HF hospitalization (transition 2). In addition, those who were hospitalized for HF were also at risk for death after a HF hospitalization (transition 3) (Figure 1). The illness-death regression model using Weibull parametrization was developed to model the effect of covariates on the cause-specific hazards of the 3-state transitions with separate (stratified) nonparametric baseline hazards for transitions into the “post-TAVI HF” state and into the “death” state. All variables associated with post-TAVI HF based on P < .05 in the univariate analyses were included in a multivariate model, together with those with clinical relevance. Hazard ratios (HR) were calculated with 95% confidence intervals (95%CI).
RESULTSBaseline characteristics, incidence, and predictors of AHF after TAVIA total of 399 patients with severe AS underwent TAVI and were included in our registry between 2008 and 2017. The mean age of the cohort was 82.4 ± 5.8 years, and 53.4% (n = 213) were women.
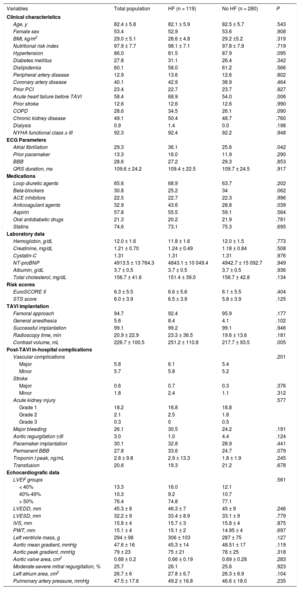
Table 1 shows the baseline characteristics of the total population and of each group, including medical history, echocardiographic features, procedural details and in-hospital outcomes.
Baseline characteristics of the total population and of each group
| Variables | Total population | HF (n = 119) | No HF (n = 280) | P |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age, y | 82.4 ± 5.8 | 82.1 ± 5.9 | 82.5 ± 5.7 | .543 |
| Female sex | 53.4 | 52.9 | 53.6 | .908 |
| BMI, kg/m2 | 29.0 ± 5.1 | 28.6 ± 4.8 | 29.2 ±5.2 | .319 |
| Nutritional risk index | 97.9 ± 7.7 | 98.1 ± 7.1 | 97.8 ± 7.9 | .719 |
| Hypertension | 86.0 | 81.5 | 87.9 | .095 |
| Diabetes mellitus | 27.8 | 31.1 | 26.4 | .342 |
| Dislipidemia | 60.1 | 58.0 | 61.2 | .566 |
| Peripheral artery disease | 12.9 | 13.6 | 12.6 | .802 |
| Coronary artery disease | 40.1 | 42.9 | 38.9 | .464 |
| Prior PCI | 23.4 | 22.7 | 23.7 | .827 |
| Acute heart failure before TAVI | 58.4 | 68.9 | 54.0 | .006 |
| Prior stroke | 12.6 | 12.6 | 12.6 | .990 |
| COPD | 28.6 | 34.5 | 26.1 | .090 |
| Chronic kidney disease | 49.1 | 50.4 | 48.7 | .760 |
| Dialysis | 0.9 | 1.4 | 0.0 | .198 |
| NYHA functional class ≥ III | 92.3 | 92.4 | 92.2 | .948 |
| ECG Parameters | ||||
| Atrial fibrillation | 29.3 | 36.1 | 25.6 | .042 |
| Prior pacemaker | 13.3 | 16.0 | 11.9 | .290 |
| BBB | 28.6 | 27.2 | 29.3 | .853 |
| QRS duration, ms | 109.6 ± 24.2 | 109.4 ± 22.5 | 109.7 ± 24.5 | .917 |
| Medications | ||||
| Loop diuretic agents | 65.6 | 68.9 | 63.7 | .202 |
| Beta-blockers | 30.8 | 25.2 | 34 | .062 |
| ACE inhibitors | 22.5 | 22.7 | 22.3 | .996 |
| Anticoagulant agents | 32.9 | 43.6 | 28.8 | .039 |
| Aspirin | 57.8 | 55.5 | 59.1 | .564 |
| Oral antidiabetic drugs | 21.3 | 20.2 | 21.9 | .781 |
| Statins | 74.6 | 73.1 | 75.3 | .695 |
| Laboratory data | ||||
| Hemoglobin, g/dL | 12.0 ± 1.6 | 11.8 ± 1.6 | 12.0 ± 1.5 | .773 |
| Creatinine, mg/dL | 1.21 ± 0.70 | 1.24 ± 0.49 | 1.18 ± 0.84 | .508 |
| Cystatin-C | 1.31 | 1.31 | 1.31 | .976 |
| NT-proBNP | 4913.5 ± 13 764.3 | 4843.1 ± 10 049.4 | 4942.7 ± 15 092.7 | .949 |
| Albumin, g/dL | 3.7 ± 0.5 | 3.7 ± 0.5 | 3.7 ± 0.5 | .936 |
| Total cholesterol, mg/dL | 156.7 ± 41.6 | 151.4 ± 39.0 | 158.7 ± 42.8 | .134 |
| Risk scores | ||||
| EuroSCORE II | 6.3 ± 5.5 | 6.6 ± 5.6 | 6.1 ± 5.5 | .404 |
| STS score | 6.0 ± 3.9 | 6.5 ± 3.9 | 5.8 ± 3.9 | .125 |
| TAVI implantation | ||||
| Femoral approach | 94.7 | 92.4 | 95.9 | .177 |
| General anesthesia | 5.6 | 8.4 | 4.1 | .102 |
| Successful implantation | 99.1 | 99.2 | 99.1 | .946 |
| Radioscopy time, min | 20.9 ± 22.9 | 23.3 ± 36.5 | 19.6 ± 13.6 | .181 |
| Contrast volume, mL | 226.7 ± 100.5 | 251.2 ± 110.8 | 217.7 ± 93.5 | .005 |
| Post-TAVI in-hospital complications | ||||
| Vascular complications | .201 | |||
| Major | 5.8 | 6.1 | 5.4 | |
| Minor | 5.7 | 5.8 | 5.2 | |
| Stroke | ||||
| Major | 0.6 | 0.7 | 0.3 | .376 |
| Minor | 1.8 | 2.4 | 1.1 | .312 |
| Acute kidney injury | .577 | |||
| Grade 1 | 18.2 | 16.8 | 18.8 | |
| Grade 2 | 2.1 | 2.5 | 1.8 | |
| Grade 3 | 0.3 | 0 | 0.5 | |
| Major bleeding | 26.1 | 30.5 | 24.2 | .191 |
| Aortic regurgitation ≥III | 3.0 | 1.0 | 4.4 | .124 |
| Pacemaker implantation | 30.1 | 32.8 | 28.9 | .441 |
| Permanent BBB | 27.8 | 33.6 | 24.7 | .079 |
| Troponin I peak, ng/mL | 2.8 ± 9.8 | 2.9 ± 13.3 | 1.8 ± 1.9 | .245 |
| Transfusion | 20.6 | 19.3 | 21.2 | .678 |
| Echocardiografic data | ||||
| LVEF groups | .561 | |||
| < 40% | 13.3 | 16.0 | 12.1 | |
| 40%-49% | 10.3 | 9.2 | 10.7 | |
| > 50% | 76.4 | 74.8 | 77.1 | |
| LVEDD, mm | 45.3 ± 8 | 46.3 ± 7 | 45 ± 9 | .246 |
| LVESD, mm | 32.2 ± 9 | 33.4 ± 8.9 | 33.1 ± 9 | .779 |
| IVS, mm | 15.8 ± 4 | 15.7 ± 3 | 15.8 ± 4 | .875 |
| PWT, mm | 15.1 ± 4 | 15.1 ± 2 | 14.95 ± 4 | .697 |
| Left ventricle mass, g | 294 ± 98 | 306 ± 103 | 287 ± 75 | .127 |
| Aortic mean gradient, mmHg | 47.6 ± 16 | 45.3 ± 14 | 48.51 ± 17 | .119 |
| Aortic peak gradient, mmHg | 79 ± 23 | 75 ± 21 | 78 ± 25 | .318 |
| Aortic valve area, cm2 | 0.68 ± 0.2 | 0.66 ± 0.19 | 0.69 ± 0.28 | .283 |
| Moderate-severe mitral regurgitation, % | 25.7 | 26.1 | 25.6 | .923 |
| Left atrium area, cm2 | 26.7 ± 6 | 27.8 ± 6.7 | 26.3 ± 6.9 | .104 |
| Pulmonary artery pressure, mmHg | 47.5 ± 17.6 | 49.2 ± 16.8 | 46.6 ± 18.0 | .235 |
ACE, angiotensin-converting enzyme; BBB, bundle branch block; BMI, body mass index; COPD, chronic obstructive pulmonary disease; ECG, electrocardiogram; IVS, interventricular septum; LVEDD, left ventricular end diastolic diameter; LVEF, left ventricular ejection fraction; LVESD, left ventricular end systolic diameter; NYHA, New York Heart Association; NT-proBNP, N-terminal pro-B-type natriuretic peptide; PCI, percutaneous coronary intervention; PWT, posterior wall thickness, STS, Society of Thoracic Surgeons; TAVI, transcatheter aortic valve implantation.
Values are expressed as percentage for categorical data and mean ± standard deviation for continuous data.
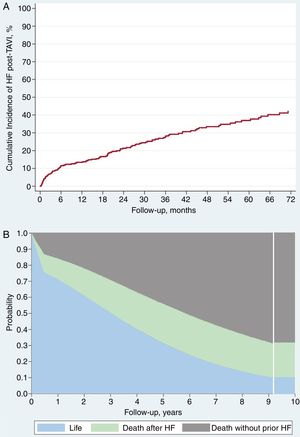
After a mean follow-up period of 27 ± 24.1 months and median of 21 months [interquartile range 6.5-40.7], 119 patients (29.82%) were admitted with a final diagnosis of AHF (cumulative incidence function 13.2%; 95%CI, 11.1%-15.8%) (Figure 2A). The average time until presentation of HF after the procedure was 20.9 ± 21.3 months with a median of 16.1 months [interquartile range 3.2-32.2]; 39.5% of AHF episodes (n = 47) occurred during the first 6 months after valve implantation.
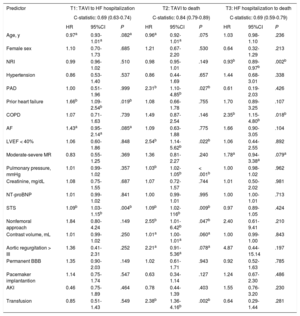
The results of the multivariate analysis are shown in Table 2. Prior to the procedure, AHF episodes and high STS score were the only independent predictors of postprocedure AHF. There was no difference between groups in left ventricular ejection fraction (not even when we stratified according to the latest HF guidelines classification)18.
Results of the multivariate analysis
| Predictor | T1: TAVI to HF hospitalization | T2: TAVI to death | T3: HF hospitalization to death | ||||||
|---|---|---|---|---|---|---|---|---|---|
| C-statistic: 0.69 (0.63-0.74) | C-statistic: 0.84 (0.79-0.89) | C-statistic: 0.69 (0.59-0.79) | |||||||
| HR | 95%CI | P | HR | 95%CI | P | HR | 95%CI | P | |
| Age, y | 0.97a | 0.93-1.01a | .082a | 0.96a | 0.92-1.01a | .075 | 1.03 | 0.98-1.10 | .236 |
| Female sex | 1.10 | 0.70-1.73 | .685 | 1.21 | 0.67-2.20 | .530 | 0.64 | 0.32-1.29 | .213 |
| NRI | 0.99 | 0.96-1.02 | .510 | 0.98 | 0.95-1.01 | .149 | 0.93b | 0.89-0.97b | .002b |
| Hypertension | 0.86 | 0.53-1.40 | .537 | 0.86 | 0.44-1.69 | .657 | 1.44 | 0.68-3.01 | .338 |
| PAD | 1.00 | 0.51-1.96 | .999 | 2.31b | 1.10-4.85b | .027b | 0.61 | 0.19-2.03 | .426 |
| Prior heart failure | 1.66b | 1.09-2.54b | .019b | 1.08 | 0.66-1.78 | .755 | 1.70 | 0.89-3.25 | .107 |
| COPD | 1.07 | 0.71-1.63 | .739 | 1.49 | 0.87-2.54 | .146 | 2.35b | 1.15-4.80b | .018b |
| AF | 1.43a | 0.95-2.14a | .085a | 1.09 | 0.63-1.88 | .775 | 1.66 | 0.90-3.05 | .104 |
| LVEF < 40% | 1.06 | 0.60-1.86 | .848 | 2.54b | 1.14-5.62b | .022b | 1.06 | 0.44-2.55 | .892 |
| Moderate-severe MR | 0.83 | 0.55-1.25 | .369 | 1.36 | 0.81-2.27 | .240 | 1.78a | 0.94-3.38a | .079a |
| Pulmonary pressure, mmHg | 1.01 | 0.99-1.02 | .357 | 1.03b | 1.02-1.05b | < .001b | 1.00 | 0.98-1.02 | .962 |
| Creatinine, mg/dL | 1.08 | 0.75-1.55 | .687 | 1.07 | 0.72-1.57 | .744 | 1.01 | 0.50-2.02 | .981 |
| NT-proBNP | 1.01 | 0.99-1.02 | .841 | 1.00 | 0.99-1.01 | .995 | 1.00 | 1.00-1.01 | .713 |
| STS | 1.09b | 1.03-1.15b | .004b | 1.09b | 1.02-116b | .009b | 0.97 | 0.89-1.05 | .424 |
| Nonfemoral approach | 1.84 | 0.80-4.24 | .149 | 2.55b | 1.01-6.42b | .047b | 2.40 | 0.61-9.41 | .210 |
| Contrast volume, mL | 1.01 | 0.99-1.02 | .250 | 1.01a | 1.00-1.01a | .060a | 1.00 | 0.99-1.00 | .843 |
| Aortic regurgitation > III | 1.36 | 0.41-2.31 | .252 | 2.21a | 0.91-5.36a | .078a | 4.87 | 0.44-15.14 | .197 |
| Permanent BBB | 1.35 | 0.90-2.03 | .149 | 1.02 | 0.61-1.71 | .943 | 0.92 | 0.52-1.63 | .785 |
| Pacemaker implantantion | 1.14 | 0.75-1.74 | .547 | 0.63 | 0.34-1.14 | .127 | 1.24 | 0.67-2.30 | .486 |
| AKI | 0.46 | 0.75-1.89 | .464 | 0.78 | 0.44-1.39 | .403 | 1.55 | 0.76-3.20 | .230 |
| Transfusion | 0.85 | 0.51-1.43 | .549 | 2.38b | 1.36-4.16b | .002b | 0.64 | 0.29-1.44 | .281 |
95%CI, 95% confidence interval; AF, atrial fibrillation; AKI, acute kidney injury; BBB, bundle branch block; COPD, chronic obstructive pulmonary disease; HR, hazard ratio; LVEF, left ventricular ejection fraction; MR, mitral regurgitation; NRI, nutritional risk index; NT-proBNP, N-terminal pro-B-type natriuretic peptide; PAD, peripheral arterial disease; STS, Society of Thoracic Surgeons; TAVI, transcatheter aortic valve implantation.
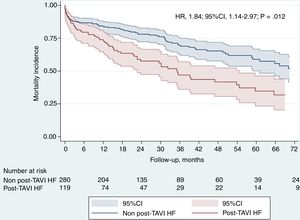
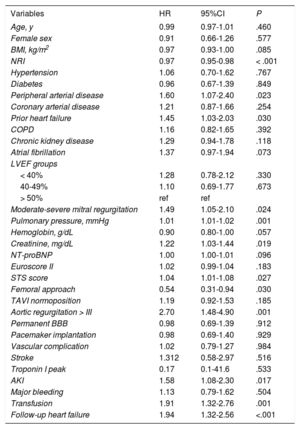
During follow-up, 150 deaths occurred in our cohort: 31 patients (20% of total deaths) during the first 30 days and 119 during the remaining follow-up (Figure 2B). The factors associated with higher mortality are summarized in Table 3 (univariate) and Table 2 (multivariate; Table 2 and Table 3 for with and without AHF during follow-up, respectively). After adjustment for these factors, AHF was a strong independent predictor of mortality (HR, 1.84; 95%CI, 1.14-2.97; P < .12), with almost twice the mortality rate in comparison with those without follow-up AHF (Figure 3).
Factors associated with higher mortality
| Variables | HR | 95%CI | P |
|---|---|---|---|
| Age, y | 0.99 | 0.97-1.01 | .460 |
| Female sex | 0.91 | 0.66-1.26 | .577 |
| BMI, kg/m2 | 0.97 | 0.93-1.00 | .085 |
| NRI | 0.97 | 0.95-0.98 | < .001 |
| Hypertension | 1.06 | 0.70-1.62 | .767 |
| Diabetes | 0.96 | 0.67-1.39 | .849 |
| Peripheral arterial disease | 1.60 | 1.07-2.40 | .023 |
| Coronary arterial disease | 1.21 | 0.87-1.66 | .254 |
| Prior heart failure | 1.45 | 1.03-2.03 | .030 |
| COPD | 1.16 | 0.82-1.65 | .392 |
| Chronic kidney disease | 1.29 | 0.94-1.78 | .118 |
| Atrial fibrillation | 1.37 | 0.97-1.94 | .073 |
| LVEF groups | |||
| < 40% | 1.28 | 0.78-2.12 | .330 |
| 40-49% | 1.10 | 0.69-1.77 | .673 |
| > 50% | ref | ref | |
| Moderate-severe mitral regurgitation | 1.49 | 1.05-2.10 | .024 |
| Pulmonary pressure, mmHg | 1.01 | 1.01-1.02 | .001 |
| Hemoglobin, g/dL | 0.90 | 0.80-1.00 | .057 |
| Creatinine, mg/dL | 1.22 | 1.03-1.44 | .019 |
| NT-proBNP | 1.00 | 1.00-1.01 | .096 |
| Euroscore II | 1.02 | 0.99-1.04 | .183 |
| STS score | 1.04 | 1.01-1.08 | .027 |
| Femoral approach | 0.54 | 0.31-0.94 | .030 |
| TAVI normoposition | 1.19 | 0.92-1.53 | .185 |
| Aortic regurgitation > III | 2.70 | 1.48-4.90 | .001 |
| Permanent BBB | 0.98 | 0.69-1.39 | .912 |
| Pacemaker implantation | 0.98 | 0.69-1.40 | .929 |
| Vascular complication | 1.02 | 0.79-1.27 | .984 |
| Stroke | 1.312 | 0.58-2.97 | .516 |
| Troponin I peak | 0.17 | 0.1-41.6 | .533 |
| AKI | 1.58 | 1.08-2.30 | .017 |
| Major bleeding | 1.13 | 0.79-1.62 | .504 |
| Transfusion | 1.91 | 1.32-2.76 | .001 |
| Follow-up heart failure | 1.94 | 1.32-2.56 | <.001 |
95%CI, 95% confidence interval; AKI, acute kidney injury; BBB, bundle branch block; BMI, Body mass index; COPD, chronic obstructive pulmonary disease; HR, hazard ratio; LVEF, left ventricular ejection fraction; NRI, nutritional risk index; NT-proBNP, N-terminal pro-B-type natriuretic peptide; ref, reference group; STS, Society of Thoracic Surgeons; TAVI, transcatheter aortic valve implantation.
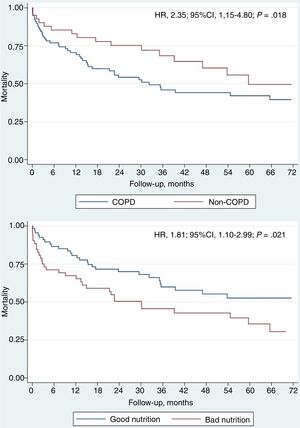
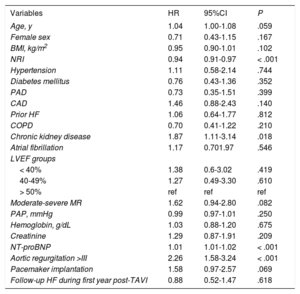
Among the 119 patients who developed HF during follow-up, 62 (52.1%) died, with an average time between the first HF event and death of 25.7 ± 16.7 months, and a median of 16.8 months [interquartile rage 7.0-35.5]. Table 4 shows the predictive factors for mortality in this patient subgroup. Univariate analysis of patients readmitted with AHF who died showed that these patients were older, with a higher rate of AF and kidney failure, were at higher risk of malnutrition (assessed by NRI), and had increased values of N-terminal pro-B-type natriuretic peptide. Statistically significant differences in echocardiographic measurements were found only for mitral regurgitation greater than moderate and aortic regurgitation grade III or higher. In the multivariate analysis (Table 2 and Table 3), we only identified reduced NRI (HR, 0.93; 95%CI, 0.89-0.97; P = .002) and chronic obstructive pulmonary disease (HR, 2.35; 95%CI, 1.15-4.80; P = .018) as variables significantly associated with higher mortality (Figure 4).
Predictive factors for mortality in the subgroup of patients developing HF during follow-up
| Variables | HR | 95%CI | P |
|---|---|---|---|
| Age, y | 1.04 | 1.00-1.08 | .059 |
| Female sex | 0.71 | 0.43-1.15 | .167 |
| BMI, kg/m2 | 0.95 | 0.90-1.01 | .102 |
| NRI | 0.94 | 0.91-0.97 | < .001 |
| Hypertension | 1.11 | 0.58-2.14 | .744 |
| Diabetes mellitus | 0.76 | 0.43-1.36 | .352 |
| PAD | 0.73 | 0.35-1.51 | .399 |
| CAD | 1.46 | 0.88-2.43 | .140 |
| Prior HF | 1.06 | 0.64-1.77 | .812 |
| COPD | 0.70 | 0.41-1.22 | .210 |
| Chronic kidney disease | 1.87 | 1.11-3.14 | .018 |
| Atrial fibrillation | 1.17 | 0.701.97 | .546 |
| LVEF groups | |||
| < 40% | 1.38 | 0.6-3.02 | .419 |
| 40-49% | 1.27 | 0.49-3.30 | .610 |
| > 50% | ref | ref | ref |
| Moderate-severe MR | 1.62 | 0.94-2.80 | .082 |
| PAP, mmHg | 0.99 | 0.97-1.01 | .250 |
| Hemoglobin, g/dL | 1.03 | 0.88-1.20 | .675 |
| Creatinine | 1.29 | 0.87-1.91 | .209 |
| NT-proBNP | 1.01 | 1.01-1.02 | < .001 |
| Aortic regurgitation >III | 2.26 | 1.58-3.24 | < .001 |
| Pacemaker implantation | 1.58 | 0.97-2.57 | .069 |
| Follow-up HF during first year post-TAVI | 0.88 | 0.52-1.47 | .618 |
95%CI, 95% confidence interval; BMI, body mass index; COPD, chronic obstructive pulmonary disease; CAD, coronary arterial disease; HF, heart failure; HR, hazard ratio; LVEF, left ventricular ejection fraction; MR, mitral regurgitation; NRI, nutritional risk index; NT-proBNP, N-terminal pro-B-type natriuretic peptide; PAD, peripheral arterial disease; ref, reference group; PAP, pulmonary artery pressure; TAVI, transcatheter aortic valve implantation.
In our TAVI registry, we gathered data from a single high-volume center expert in TAVI and included 399 TAVI patients. To our knowledge, this is the first study that assesses the incidence, prognostic impact, and predictive factors for hospital admission due to AHF following TAVI with the CoreValve device. The main findings of our study are as follows: a) there was a high incidence of AHF episodes requiring hospital admission, most of them with preserved ejection fraction, and up to 5-years of follow-up; b) those patients who developed AHF after TAVI had higher mortality and were associated with pre-TAVI hospital admissions for AHF and high STS score, and c) poor NRI and chronic obstructive pulmonary disease were independent mortality predictors in patients who developed AHF.
Incidence of hospital admission due to AHFSeveral studies and registries have shown a high readmission rate in patients after TAVI.19 The rate of early (within 30 days) readmission ranged from 4.0% to 17.9%, and was higher in those patients who underwent TAVI through a transapical approach. The readmission rate during the first year post-TAVI was as high as 50% of patients, mostly for noncardiovascular causes. A study by Nombela-Franco et al.20 reported an incidence of 43.9% for all-cause readmissions up to 1 year after TAVI. Among them, 58.9% were admitted for noncardiovascular causes (mainly due to pre-existing comorbidities) and 41.1% for cardiac causes, mainly AHF (23.3%). Similar results were reported by Durand et al., 21 with 1-year total readmission rate and for AHF of 52.2% and 24.1%, respectively. Of note, in both studies the valve used was the SAPIEN device. Our registry, which mainly examined patients receiving the CoreValve device, shows similar results: 30% of our population was admitted due to AHF during follow-up, less than a half of these admissions being during the first year after the procedure (46.2%).
Prognostic impact of hospital admission due to AHFAt the end of follow-up, 150 patients had died (37.59%). Our results are similar to previous published data by Avanzas et al.22: that group also remarked on the relevance of admission due to HF in TAVI patients, highlighting 92.6% of deaths after an admission. There are scarce reports on the determinants and prognosis of AHF after TAVI. As far as we know, the study by Durand et al.21 was pioneer in reporting the impact of AHF on mortality after TAVI. Among patients discharged from hospital, the rate of all-cause mortality was 13.7% at 1-year, and was 31.4% after a mean follow-up period of 27.2 + 0.7 months. Readmission due to AHF after TAVI was strongly associated with higher mortality at 1 year (24.2% vs 10.4%, P < .0001) and at the end of follow-up (50.0% vs 25.6%, P < .0001). Our results are fairly similar. After a mean follow-up period of 27 ± 24.1 months, mortality in patients admitted due to AHF was 52.1% vs 31.4% (HR, 1.84; 95%CI, 1.14-2.97; P < 0012). Nombela-Franco et al.20 also assessed the impact of early hospital admission on mortality, with a mean follow-up similar to that one in our study. Their reported mortality rate at 2 years was significantly increased in those patients who were admitted within 30 days after TAVI compared with those who were not readmitted (30.2% vs 19.2%; P = .002). Approximately 30% to 50% of the readmissions were related to cardiovascular causes, mostly AHF, and had a major prognostic impact on mortality.
Predictive factors for hospital admission for AHFTwo independent factors were identified as predictors for AHF after the index discharge: an episode of hospital admission for AHF before TAVI implantation and high risk measured by STS score. Durand et al.21 found 4 independent predictors for AHF: low aortic mean gradient before TAVI, postprocedural blood transfusion, severe persistent postprocedural pulmonary hypertension, and left atrial dilatation, of which only 2 were procedure-related. In this study, a previous episode of AHF before TAVI failed to achieve statistical significance in the multivariate analysis. Baron et al.23 reported the impact of aortic valve gradient on the outcomes of TAVI in a large series of patients (n = 11 292); low aortic valve gradient (< 40mmHg) was associated with higher mortality (HR, 1.21; 95%CI, 1.11 - 1.32; P < .001) and higher rates of AHF (HR, 1.52; 95%CI, 1.36-1.69; P < .001) with no effect of LVEF. We observed no significant impact for pre-TAVI aortic valve gradient or for LVEF. These discrepancies may be explained by demographic and clinical differences between the populations.
In our registry, despite pulmonary arterial pressure being higher in the group that developed AHF after TAVI, it did not have an impact on prognosis in our cohort. Several publications showed that pulmonary hypertension before TAVI is frequent and increases mortality after TAVI.24,25 Patients with persistent severe pulmonary hypertension after TAVI have worse prognosis than those with a decrease in pulmonary artery systolic pressure below 60mmHg (2-year mortality rate 50.0% vs 18.6%, P = .001). It was postulated that right heart catheterization could therefore aid in Heart Team decision-making.
Major bleeding and transfusion is frequent following TAVI and was associated with an increased risk of early and late mortality.26 Durand et al.21 observed that severe bleeding and, particularly, the need for transfusions were independent predictors of admission due to AHF after TAVI. In our cohort the need for transfusions was not associated with increased AHF, but it was a marker of higher mortality after implantation.
Predictive factors for mortality in patients with AHF: the role of NRINRI and chronic obstructive pulmonary disease after valve implantation were found to be key elements influencing the prognosis of this group of patients. Our results show for the first time how nutritional status assessed by using NRI is a powerful independent prognostic factor. NRI is a validated tool for estimating the risk of undernutrition in various populations. The NRI shows a strong correlation with mortality, adverse events and deterioration of functional capacity, which is superior to that achieved using body mass index and albumin separately.27–30 Malnutrition is highly prevalent and has been reported to be an independent risk factor for clinical events in HF. A large proportion of patients hospitalized for HF have moderate to severe malnutrition, and low NRI is associated with more readmissions and higher mortality in patients with AHF, as well as with higher mortality in patients with chronic HF.27–30 A relationship between classic nutritional status markers, such as body mass index and hypoalbuminemia, and prognosis after TAVI (with a J-shape curve) has been established in previous studies.31
Assessment of malnutrition risk must be part of the geriatric assessment of patients who undergo TAVI and plays a determining role in frailty status. The potential use of the NRI for early identification of patients at risk of malnutrition who are under assessment for TAVI could be highly relevant in daily clinical practice. Such patients could potentially benefit from interventions to improve their nutritional status prior to undergoing the procedure. Larger studies are needed to validate NRI within a geriatric and frailty score, alone or as a part of other predictive scores for events after TAVI.
Chronic obstructive pulmonary disease is common in HF patients, and its presence in those with systolic dysfunction is associated with an increased burden of comorbidities, lower use of evidence-based HF medications, longer hospital stays, and increased in-hospital noncardiovascular mortality.32
CONCLUSIONSThe proportion of patients who develop AHF after TAVI is high, and prior AHF status has an important prognostic significance. There is a need to identify subgroups of patients at higher risk in order to optimize their status prior to the procedure. In our study, patients with a previous history of AHF and high STS score were more prone to develop AHF during follow-up. Closer surveillance of these patients, with targeted and intensive medical treatment, could be helpful to try to reduce readmissions due to HF and improve their prognosis. In view of our results, intervention programs on the nutritional status of patients who develop AHF after TAVI and who are at risk of malnutrition could also improve their survival. In our study, as in previous studies, few factors have been identified as predictors of AHF. Future efforts should focus on the search for more predictors of AHF and evaluate whether an intervention during follow-up, such as optimization of medical therapy or nutritional status, has an impact on the prognosis of this subgroup of patients.
Conflicts of interestR. Trillo Nouche is a proctor for Medtronic.
- –
The evidence on HF after TAVI is scarce. Previous studies have observed the adverse impact of HF on post-TAVI prognosis, but the number of well recognized predictive factors is still very low.
- –
To our knowledge, this is the study to assess the incidence, prognostic impact, and predictive factors for hospital admission due to AHF following TAVI with the CoreValve device.
- –
In our cohort, 2 potent prognostic determinants were found: a previous history of AHF and high STS score.
- –
Our results also show for the first time how nutritional status, assessed using NRI, is a powerful independent prognostic factor in this subgroup of patients, suggesting that this index could be included in the evaluation of candidates for TAVI.
To Manuela Sestayo for her outstanding help with language editing.