Keywords
INTRODUCTION
The clinical study of ventricular fibrillation (VF) has always been impeded by its unpredictable occurrence and by the short time periods the patient can cope with it. Consequently, VF signal recordings are complicated and difficult to obtain and references in the literature to spontaneous VF with electrocardiogram (ECG) records are limited.1 Recently, the successful ablation of premature ventricular complexes (PVCs) that trigger VF episodes in arrhythmic storm has been reported.2-7 These cases stimulate our interest in understanding the mechanism that triggers VF. Implantable cardioverter-defibrillators (ICDs) enable us to store data on arrhythmic episodes and analysis of this data means our study of VF-trigger mechanisms can progress. To study the mechanism underlying the initiation of isolated VF or VF recurrence in arrhythmic storm, we analyzed ICD-recorded data of patients experiencing spontaneous VF episodes.
METHODS
We analyzed the VF records (near-field and far-field) of 250 patients with ICD implants received at a tertiary hospital between 1997 and 2008. Of these, 13 presented ≥1 VF episode. The ICDs analyzed belonged to the MINI and PRIZM series (Guidant, St. Paul, Minnesota, USA) and the GEM series (Medtronic, Minneapolis, Minnesota, USA). All ICDs were programmed to record and store the signal prior to the declared VF episode. We defined as VF those episodes with sinusoidal electrograms, with changeable morphology, cycle and width in which clearly-defined QRS complexes could not be discerned, and with true or pseudo-bipolar records with a characteristically fragmented signal. All episodes required electric discharge to finalize them, and we rejected those that ended of their own accord. We considered these were examples of polymorphous ventricular tachycardia and excluded them from the study. We defined the short-long-short cycle as the appearance of a (short) premature beat in the baseline rhythm, followed by a pause (with coupling interval >1.2-fold the preceding interval) and premature depolarization with (short) short coupling that triggers VF.
We analyzed morphology in the near-field and far-field channel, coupling interval, preceding sinus beat cycle, and the presence of a short-long-short sequence preceding premature beats that did (fibrillatory PVC) and did not (non-fibrillatory PVC) trigger VF. We used the paired Student t test to compare the coupling interval and the preceding sinus beat cycle length of both premature beats.
RESULTS
We recorded 31 episodes of spontaneous VF in 13 patients (10 men and 3 women; age, 49 [22] years). Patient diagnoses were: 4, Brugada syndrome; 3, ischemic heart disease; 1, hypertrophic cardiomyopathy; 2, dilated cardiomyopathy; 1, short-coupled variant of torsades de pointes; 1, endocardial fibroelastosis; and 1 idiopathic VF. Implantation was indicated for VF in 7 patients: 2 had ischemic heart disease; 2, Brugada syndrome; 1, hypertrophic cardiomyopathy; 1, endocardial fibroelastosis; and 1, short-coupled variant of torsades de pointes. We recorded a mean of 2.5 episodes/patient (1-12). Of 31 VF episodes, 14 were isolated and the rest were related with arrhythmic storm. Three patients presented arrhythmic storm. All episodes analyzed began with PVC, which was of the same morphology and had a similar coupling interval in the 7 patients with >1 episode (time between episodes ranging from minutes to 3 years) (Figures 1 and 2). The premature beat coupling interval range was 409 (121) ms and the preceding sinus beat cycle was 801 (233) ms. We found long-short cycle occurrence was related with VF onset in only 2 cases (9%). In 21 episodes, VF was preceded by PVC in sinus rhythm that did not trigger VF. The morphology, coupling interval (411 [123] ms) and preceding sinus beat cycle (793 [230] ms) presented no significant differences with respect to the PVC that triggered VF.
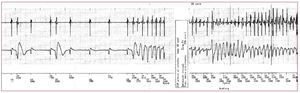
Figure 1. Onset of an episode of ventricular fibrillation in a patient with short-coupled variant of torsades de pointes.
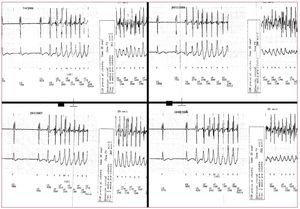
Figure 2. Onset of four episodes of ventricular fibrillation on different days corresponding to a patient with Brugada syndrome. Note the similar morphology of the premature ventricular complexes that trigger ventricular fibrillation.
DISCUSSION
The PVC that precedes VF initiation has been known for some time and instances of primary VF in acute myocardial infarction have been described.8 More recently, this has been believed to be the mechanism that triggers VF episodes in arrhythmic storm, and VF recurrence has been avoided by using ablation to eliminate it.2-7
In the pioneering studies of causes of ambulatory sudden cardiac death,1 VF preceded by PVC only accounted for 8.28% and the vast majority of VF episodes (83.4%) were triggered by ventricular tachycardia. The magnitude of the difference between these results and those we obtained may be explained by the use of different methods of recording data. Bayés et al1 reported on data collection by Holter monitoring; our study involved ICD recordings. We assume that in our patients possible episodes of ventricular tachycardia that might have degenerated into VF were successfully treated by ICD anti-tachycardia therapy, which has proven effective in a high percentage of ventricular tachycardia.9,10
In contrast to the onset of monomorphic ventricular tachycardia, which has been studied extensively in ICD recipients,11 few studies have analyzed VF initiation.12,13 Among those that have, research in MADIT (Multicenter Automatic Defibrillator Implantation Trial) II-type patients, a study of primary prevention in patients with ischemic heart disease and ventricular dysfunction, stands out.12 This study found that PVCs triggered 77% of VF episodes, in contrast to a short-long-short sequence found in 23% of patients (14 episodes out of 60).
In patients with Brugada syndrome, Kakishita et al14 described PVC-triggered VF and included a figure that showed three episodes of VF, on three different dates, in which PVC morphology was markedly similar. We found the same consistency in VF-inducing PVC characteristics in our patients.
Although the pre-VF time recorded is brief, during non-VF inducing PVCs with similar morphologic and electric characteristics in terms of the coupling interval, we have found we can distinguish between fibrillatory and non-fibrillatory premature beats that coincide in a short period of time. The registry of ambulatory sudden cardiac death also describes a significant increase in the number of PVCs in the hour before VF.1
We do not know why some premature beats trigger VF and others, with no obvious electrophysiological differences, do not. The fact that the PVC that triggers VF may at different times in the natural history of the patient have the same morphology, indicates that in each patient the premature beats that induce VF originate in the same way. This could be of interest in targeted VF ablation procedures, if we assume that it could be induced.
Limitations
The most important limitation of this study is the small number of patients included, which is partly due to the low incidence of spontaneous VF episodes.
Similarly, as only two channels are available (near-field and far-field) to analyze the episodes, difficulties in differentiation could arise in ventricular tachycardia fibrillation episodes with very fast cycles. The low number of long-short sequence initiated episodes means comparison of the two onset types' characteristics (coupling, preceding QT interval, T wave width and prior heart rate) is impossible.
Furthermore, the morphologic discrimination capability of the ICDs' stored memory channels prevents us from discounting the possibility that VF-inducing PVCs may differ slightly in VF recurrence episodes.
CONCLUSIONS
Spontaneous VF is triggered by PVCs both in cases of arrhythmic storm and in isolated episodes. Premature beats often precede VF but do not induce it. All this indicates that the VF-trigger mechanism has specific characteristics in each patient.
ABBREVIATIONS
ICD: implantable cardioverter-defibrillator
PVC: premature ventricular complex
VF: ventricular fibrillation
This manuscript was part-financed by Spanish Ministry of Education and Culture research project TEC2007-68096-C02-TCM.
Some results from this study were presented at the XIII World Congress on Cardiac Pacing and Electrophysiology, Rome, Italy 2007 and published as an abstract in Giornale Italiano di Aritmologia e Cardiostimulazione. 2007;10(3):109.
Correspondence: Dr J.J. Sánchez-Muñoz.
Unidad de Arritmias. Servicio de Cardiología. Hospital Universitario Virgen de la Arrixaca.
Ctra. Madrid-Cartagena, s/n. 30120 El Palmar. Murcia. Spain.
E-mail: juanjosanchezmunoz@me.com
Received December 14, 2009.
Accepted for publication January 25, 2010.