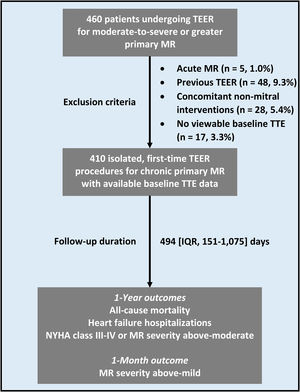
Limited data exist on the prognostic usefulness of transthoracic echocardiography preceding MitraClip for chronic primary mitral regurgitation (MR). We evaluated the predictive ability of transthoracic echocardiography in this setting.
MethodsA total of 410 patients (median age, 83 years, 60.7% males) were included in the study. The primary outcome was the 1-year composite of all-cause mortality or heart failure hospitalization. Secondary endpoints encompassed individual elements of the primary outcome, the persistence of significant functional impairment or above-moderate MR at 1 year, and above-mild MR at 1-month.
ResultsThe only parameter associated with the risk of the primary outcome was a ventricular end systolic diameter index of ≥2.1 cm/m2, corresponding to the cohort's 4th quartile (HR, 2.44; 95%CI, 1.09-4.68; P=.022). Concurrently, higher left atrial volume index (LAVi) and a mid-diastolic medial-lateral mitral annular diameter (MAD) equal to or above the cohort's median of 32.2mm were linked to a higher probability of death and heart failure hospitalization, respectively. LAVi of ≥ 60mL/m2, above-mild mitral annular calcification, and above-moderate tricuspid regurgitation conferred higher odds of functional class III-IV or above-moderate MR persistence. All variables except LAVi and MAD, as well as indexed mid-diastolic medial-lateral MAD of ≥ 20.2mm/m2 and mitral effective regurgitant orifice area of ≥ 0.40 cm2, were associated with greater-than-mild MR at 1 month.
ConclusionsPreprocedural increased indexed left heart dimensions, mainly left ventricular end-systolic diameter index, MAD, mitral annular calcification, mitral effective regurgitant orifice area, and tricuspid regurgitation mark a less favorable course post-MitraClip for chronic primary MR.
Keywords
Transcatheter edge-to-edge repair (TEER) is a well-established treatment for chronic primary mitral regurgitation (PMR).1 While associated with excellent short-term structural results, the procedure is challenged by less than optimal clinical outcomes, which could theoretically be optimized by improved patient selection. A readily available, noninvasive, and highly standardized imaging modality, transthoracic echocardiography (TTE) plays a key role in screening mitral TEER candidates and may prove useful for risk stratification purposes as well. To date, however, the prognostic value of preprocedural TTE in the setting of TEER for chronic PMR has not been substantiated.
Previous studies exploring the utility of imaging studies in the triage of patients considered for TEER have either focused on transesophageal echocardiographic (TEE) parameters,2 evaluated only a few TTE variables simultaneously, incorporated clinical characteristics in the analyses,3 and/or been performed in functional4–9 or heterogenous MR10–15 cohorts. Similarly, imaging factors included in current outcome prediction models for mitral TEER have not been validated exclusively in the chronic PMR population.16–19 To address this knowledge gap, we assessed the prognostic significance of common TTE parameters obtained prior to TEER for chronic PMR, using the data of a large, real-world registry.
METHODSData availabilityThe data used in this article will be shared upon reasonable request to the corresponding authors.
Study population and outcomesOur study represents a retrospective analysis of the Cedars-Sinai database of consecutive TEER procedures performed between January 1, 2013 and January 1, 2021 in adult patients for moderate-to-severe or greater MR accompanied by myocardial dysfunction and/or symptoms despite maximally tolerated medical therapy. Each intervention was undertaken following a Heart Team discussion, which considered overall patient status as judged clinically, standardized/formal surgical risk and operability, published scientific evidence, and patient preferences.
The inclusion criteria for the study were as follows: a) a diagnosis of chronic PMR, based on a morphologically abnormal valve apparatus as assessed during the intraprocedural TEE; b) the performance of an isolated, first-ever TEER; and c) the availability of a viewable preprocedural TTE.
The primary outcome was the composite of all-cause mortality or heart failure (HF) hospitalization during the first postprocedural year. Secondary endpoints included individual components of the primary outcome, as well as the persistence of significant functional impairment at 1 year, indicated by a New York Heart Association (NYHA) class III-IV, or above-moderate MR. A greater-than-mild MR at 1 month was also examined.
The study conformed to the Declaration of Helsinki and was approved by the Cedars-Sinai Institutional Review Board, which waived the need for informed consent.
Procedural aspectsMitraClip (Abbott Vascular Inc, United States) was the sole system employed in the registry. All procedures were performed under general anesthesia and used a trans-septal approach and femoral venous access. TEE, fluoroscopy, and right heart catheterization were used for guidance and monitoring. Technical success was defined as actual device deployment not accompanied by surgical intervention or major complications within the first 24hours.20
Echocardiographic assessmentEchocardiograms were performed and interpreted by experienced sonographers and level III-trained echocardiologists, following accepted guidelines.21–23 The ultrasound system used was EPIQ (Philips, United States). Postprocessing used PICOM365 (SciImage, United States), QLAB 12.0 (Philips, United States), and TomTec Arena (TomTec Imaging Systems, Germany) for 2-dimensional (2D), 3-dimensional (3D), and speckle-tracking measurements, respectively.
All parameters were evaluated by multiple, focused, and zoomed views. For each continuous structural variable, a body surface area-indexed value was calculated. Regarding hemodynamic variables, either the highest or averaged values were considered based on rhythm regularity. To ensure reliability and consistency, all baseline continuous parameters were assessed by 2 study members (A. Shechter and M. Lee), who were blinded to patient history. In addition, selected mitral and left ventricular (LV) parameters were compared with those obtained by intraprocedural TEE and preprocedural cardiac computed tomography (CCT) exams, respectively. The latter were performed in patients simultaneously considered for valve replacement.
Mitral valve (MV)-related echocardiographic parameters included regurgitation severity, transmitral mean pressure gradient (TMPG), peak E wave velocity, presence and extent of mitral annular calcification (MAC), leaflet calcification, mitral annular diameter (MAD), and leaflet tethering/restriction. MR severity was evaluated by integration of qualitative and quantitative measures and graded as 0 (up-to-minimal), 1 (mild/mild-to-moderate), 2 (moderate), 3 (moderate-to-severe), or 4 (severe). The peak E wave velocity and TMPG were inferred from pulsed-wave (PW) or continuous-wave (CW) mitral inflow tracings, respectively. MAC was assessed semiqualitatively and described as above-mild when involving more the one-third of the annular circumference on the parasternal short axis view24 or protruding into the LV on apical views. Leaflet immobility was quantified based on leaflet closing angles on the parasternal long axis (PLAX) view. Anterior-posterior (AP) and medial-lateral (M-L) MAD lengths were measured at mid- and end-diastole in the PLAX and apical 4-chamber views, respectively.
Non-MV-related variables consisted of chamber function and dimensions, concomitant valvulopathies, pulmonary arterial systolic pressure (PASP), and LV global longitudinal strain (LVGLS). Left ventricular ejection fraction (LVEF) and left heart chamber volumes were calculated using the Simpson biplane method of disks, whereas global right ventricular (RV) function was assessed qualitatively. LV mass index was computed using the American Society of Echocardiography formula. Tricuspid annular plane systolic excursion (TAPSE) corresponded to the vertical displacement of the lateral tricuspid annular edge according to M-mode tracing in the apical 4-chamber view. Tricuspid regurgitation (TR) was quantified in a similar fashion to MR. PASP was calculated by combining the maximal CW-derived TR pressure gradient with the estimated right atrial pressure; the latter was dictated by inferior vena cava diameter and collapsibility as revealed in the subcostal views. LVGLS was calculated semiautomatically following manual adjustments of cardiac cycle and tracing borders as needed, by averaging endocardial strain measurements in the apical windows.
Intraprocedural pulmonary venous flow pattern (PVFP) improvement and normalization required any increase or the emergence of a value of ≥ 1, respectively, in the peak systolic/diastolic velocity ratio on any PV by PW interrogation.
Data collectionPatient assessment was carried out at baseline, hospital discharge, and at 1 month and 1 year postprocedure. Data were extracted from an electronic medical chart, which was updated in real-time by medical providers and state authorities.
Statistical analysisVariables are reported as frequencies and percentages or medians [interquartile ranges]. Selected continuous variables were assessed for correlation and change over time using the Pearson r coefficient and Wilcoxon test, respectively. Interobserver reliability regarding continuous TTE parameters was evaluated by the intraclass correlation coefficient (ICC).
To identify associations with outcomes, Cox and binary logistic regression multivariable analyses were performed that incorporated baseline TTE parameters with perceived or previously proven3,17 prognostic significance and a P value of <.1 in univariable models. Continuous variables were assessed both as such and as dichotomous, using the medians and 1st/4th quartiles of the cohort, as well as guideline-cited thresholds for intervention.25,26 Both LVGLS-inclusive/exclusive models were constructed.
The cumulative incidence of the primary outcome and its separate components as a function of TTE parameters identified by the regression models was further analyzed by the log-rank test and was graphically displayed using the Kaplan-Meier method.
To address potential confounders encountered by the “echo-only” regression models, “comprehensive” models were constructed for the primary outcome and its elements. In addition to preprocedural TTE parameters, these included baseline clinical parameters and procedural features showing differing frequencies among patients with and without TTE findings associated with the risk of the primary outcome (all as determined by the Pearson chi-square, Fisher exact, or Mann-Whitney U tests), as well as the year of TEER performance, device generation, and data availability regarding 1-month MR grade.
Cases with missing values were censored from the relevant calculations. Statistical significance was defined as a 2-sided P value of <.05. All analyses were performed using SPSS 24 (IBM Corporation, United States).
RESULTSBaseline characteristics of the study populationA total of 410 patients were included in the analysis and followed up for a median of 494 [151-1075] days (figure 1). These patients were characterized by a median age of 83 [76-88] years, a predominance of male sex (n=249, 60.7%), and a high burden of comorbidities, mostly hypertension (table 1). HF was highly symptomatic, as shown by NYHA class III-IV in 377 (91.9%) patients. Reflecting this profile, interventional risk was medium-to-high.
Baseline clinical characteristics
| Total cohort (n=410) | |
|---|---|
| Demographic details | |
| Age | |
| Median, y | 83 (76-88) |
| ≥75 y | 318 (77.6) |
| Male sex | 249 (60.7) |
| Body surface area (Mosteller formula), m2 | 1.77 [1.59-2.00] |
| Comorbidities | |
| Obesity, body mass index ≥ 30 kg/m2 | 53 (12.9) |
| Diabetes mellitus | 77 (18.9) |
| Hypertension | 332 (81.2) |
| Smoking | 12 (2.9) |
| Chronic obstructive pulmonary disease | 50 (12.2) |
| Anemia | 238 (58.0) |
| Stage ≥ III chronic kidney disease | 309 (76.3) |
| Previous MI, PCI, or CABG | 120 (29.3) |
| Prior stroke or transient ischemic attack | 52 (12.7) |
| Peripheral arterial disease | 35 (8.6) |
| Atrial fibrillation/flutter | 220 (53.7) |
| Heart failure features | |
| New York Heart Association class | |
| II | 33 (8.0) |
| III | 176 (42.9) |
| IV | 201 (49.0) |
| KCCQ12 score, points | 42.2 [20.8-66.2] |
| 6-minute walk test distance, m | 244 [150-335] |
| Serum B-type natriuretic peptide, pg/mL | 328 [175-639] |
| Procedural risk | |
| STS score for mitral valve repair | 5.2 [2.9-8.0] |
| Mitral regurgitation international database score | 9 (8-10) |
| MitraScore | 3 (2-4) |
| Treatment | |
| Medications | |
| Beta-blockers | 250 (61.0) |
| Renin angiotensin system inhibitors | 186 (45.4) |
| Mineralocorticoid receptor antagonists | 44 (10.7) |
| Loop diuretics | 283 (69.0) |
| Antiarrhythmics | 68 (16.6) |
| Antiplatelets | 231 (56.3) |
| Oral anticoagulants | 182 (44.) |
| Cardiac implantable electronic device | |
| Total | 69 (16.8) |
| Any defibrillator device | 17 (4.1) |
| Any pacemaker device | 62 (15.1) |
CABG, coronary artery bypass grafting; KCCQ, Kansas City Cardiomyopathy Questionnaire; MI, myocardial infarction; PCI, percutaneous coronary intervention; STS, Society of Thoracic Surgeons.
Data are presented as No. (%) or median [interquartile range].
Baseline TTE, performed 25 [IQR, 8-54] days prior to the procedure, demonstrated severe MR in most of the patients (n=349, 85.5%) (table 2). Overall, MR was attributed to degenerative disease in 403 (98.3%) patients, annular/leaflet calcification in 6 (1.5%), and a combination of the 2 in 1 (0.2%). Almost half of the patients (n=195, 47.6%) had an LVEF of ≤ 60% or an LV end-systolic diameter (LVESD) of ≥ 4.0cm. A similar proportion exhibited a left atrial volume index (LAVi) of ≥ 60mL/m2, and a little more than a third had MAC and a PASP of> 50mmHg. The median LVGLS was −15.9 [−18.9-(−12.3)]%. Importantly, the interobserver reliability was good for all continuous parameters (ICC> 0.87, P <.001). In addition, mid-diastolic MAD correlated with its end-diastolic counterpart, TEE parallel, and TEE 3D MV area (Pearson r ≥ 0.72, P <.001). CCT, undertaken in 79 (19.3%) individuals within 0 [IQR, 0-8] days of the preprocedural TTE, revealed higher indexed LV volumes that nevertheless correlated with the echocardiographic observations (Pearson r ≥ 0.77, P <.001).
Baseline echocardiographic data
| Total cohort (n=410) | |
|---|---|
| Mitral valve | |
| Preprocedural transthoracic echocardiography | |
| Mitral regurgitation severity | |
| Moderate-to-severe | 59 (14.5) |
| Severe | 349 (85.5) |
| Mitral effective regurgitant orifice area by PISA, cm2 | 0.40 [0.28-0.52] |
| Mitral regurgitant volume by PISA, mL | 55.3 [41.9-77.2] |
| Transmitral mean pressure gradient, mmHg | 3 (2-4) |
| wave peak velocity, cm/sec | 126 [105-149] |
| Mitral annular calcification | |
| Any | 149 (36.3) |
| Above-mild | 53 (12.9) |
| Mitral leaflet calcification | 109 (27.4) |
| Mitral annular diameter at mid-diastole | |
| Anterior-posterior, mm | 28.9 [25.4-32.7] |
| Index, mm/m2 | 16.0 [14.1-18.6] |
| Medial-lateral, mm | 32.2 [28.7-36.0] |
| Index, mm/m2 | 17.8 [15.6-20.2] |
| Mitral annular diameter at end-diastole, mm | |
| Anterior-posterior | 26.9 [21.4-30.9] |
| Medial-lateral | 29.2 (24.8-33.1] |
| Mitral leaflet tethering/restriction | 22 (5.4) |
| Anterior leaflet closing angle, degrees | 37 [35-42] |
| Posterior leaflet closing angle, degrees | 46 [45-53] |
| Intraprocedural transesophageal echocardiography | |
| Mitral leaflet prolapse and/or flail | |
| Any | 403 (98.3) |
| Anterior | 10 (2.4) |
| Posterior | 306 (74.6) |
| Bileaflet | 87 (21.2) |
| Maximal prolapse height, mm | 6.0 [5.0-8.0] |
| Medial-lateral mitral annular diameter at mid-diastole, mm | 33.7 [29.5-38.5] |
| Index, mm/m2 | 19.0 [16.0-22.0] |
| Mitral valve area by 3-dimensional planimetry, cm2 | 5.5 [4.3-6.8] |
| Left heart | |
| Left ventricular ejection fraction | |
| Median, % | 63 [56-68] |
| ≤ 60% | 180 [43.9] |
| <20% | 2 (0.5) |
| Left ventricular end-systolic diameter | |
| Median, cm | 3.2 [2.8-3.8] |
| ≥ 4.0cm | 80 (19.5) |
| Index, cm/m2 | 1.8 [1.5-2.1] |
| Left ventricular ejection fraction ≤ 60% orLeft ventricular end-systolic diameter ≥ 4.0 cm | 195 (47.6) |
| Left ventricular end-diastolic diameter | |
| Median, cm | 5.0 [4.5-5.5] |
| Index, cm/m2 | 2.8 [2.5-3.1] |
| Left ventricular end-systolic volume | |
| Median, mL | 33.0 [22.8-48.3] |
| Index, mL/m2 | 18.5 [13.1-25.8] |
| Left ventricular end-diastolic volume, mL | |
| Median, mL | 92.0 [65.0-120.0] |
| Index, mL/m2 | 50.7 [38.4-64.7] |
| Left ventricular mass index, ASE formula, g/m2 | 117.2 [92.5-140.9] |
| Left atrial volume index | |
| Median, mL/m2 | 60.0 [44.0-76.0] |
| ≥ 60, mL/m2 | 193 (47.1) |
| Moderate of greater aortic stenosis/regurgitation | 32 (7.8) |
| Right heart | |
| Right ventricular dysfunction | |
| Any | 100 (27.1) |
| Moderate/severe | 39 (10.6) |
| Basal right ventricular diameter at end-diastole, cm | 4.0 [3.5-4.4] |
| Above-moderate tricuspid regurgitation | 76 (18.6) |
| Right ventricular-pulmonary arterial coupling | |
| TAPSE, mm | 18 (15-22) |
| PASP | |
| Median, mmHg | 43 (33-57) |
| > 50 mmHg | 159 (38.8) |
| > 70 mmHg | 41 (10.0) |
| TAPSE/PASP, mm/mmHg | 0.41 [0.29-0.61] |
| Speckle-tracking | |
| Left ventricular global longitudinal strain, % | −15.9 [−18.9-(−12.3)] |
ASE, American Society of Echocardiography; PASP, pulmonary arterial systolic pressure; PISA, proximal isovelocity surface area; TAPSE, tricuspid annular plane systolic excursion
Data are presented as No. (%) or median [interquartile range].
Most procedures employed 1 to 2 first/second-generation devices and targeted the A2P2 segment (table 1 of the supplementary data). Almost all (n=401, 97.8%) were completed successfully, allowing for next-day discharge in most patients.
Immediately following clip deployment, MR regressed to mild-or-less in 317 (77.3%) patients and PVFP improved in 300 (85.5%). Later in the first postprocedural year, both LVEF, left heart dimensions, and PASP significantly decreased (table 2 of the supplementary data).
OutcomesBy 1 year, 61 (14.9%) patients had experienced the primary outcome of all-cause mortality (n=35, 8.5%) or HF hospitalizations (n=37, 9.0%). Of the 375 patients who survived to the first postprocedural year, 217 (57.9%) remained under active surveillance at Cedars-Sinai and had available data relating to functional status or residual MR (functional status, n=204/375, 54.4%; residual MR, n=184/375, 49.1%). Among the latter, 40 (18.4%) were found to be in NYHA class III-IV (n=24/204, 11.8%) or to demonstrate above-moderate MR (n=19/184, 10.3%) at 1 year. One-month MR grade, which as a continuous variable directly correlated with its 1-year counterpart (Pearson r=0.75, P <.001), was above-mild in 115 (36.6%) of the 314 individuals with viewable echocardiograms, the latter comprising 77.5% (n=314/405) of patients remaining alive at 1 month.
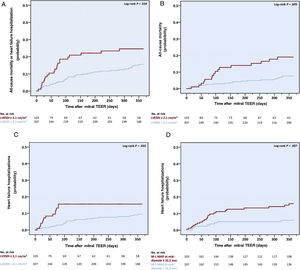
Baseline echocardiographic parameters associated with the outcomesAfter multivariable analysis, an LVESD index (LVESDi) of ≥ 2.1cm/m2, corresponding to the 4th quartile of the cohort, emerged as the only preprocedural TTE parameter that was associated with the risk of the primary outcome, more than doubling its risk (hazard ratio [HR], 2.44; 95% confidence interval [95%CI], 1.09-4.68; P=.022) (table 3 of the supplementary data and table 3). An LVESDi of ≥ 2.1cm/m2 also conferred a higher probability of all-cause mortality (HR, 2.17, 95%CI, 1.28-4.88; P=.020) and HF hospitalizations (HR, 3.27; 95%CI, 1.38-5.75; P=.007) in separate analyses (tables 4 and 5 of the supplementary data), and was associated with higher rates and cumulative incidences of all above-mentioned outcomes (figure 2, table 6 of the supplementary data, and figure 1 of the supplementary data). Of note, the univariate link between increased LVESD and the cumulative incidence of the primary outcome was observed regardless of baseline LVEF and LVESD intervention cutoffs (figures 2 and 3 of the supplementary data).
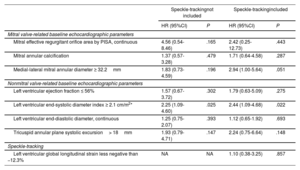
Multivariable echocardiography-only cox proportional hazard model for the composite outcome of all-cause mortality or heart failure hospitalization at 1 year
| Speckle-trackingnot included | Speckle-trackingincluded | |||
|---|---|---|---|---|
| HR (95%CI) | P | HR (95%CI) | P | |
| Mitral valve-related baseline echocardiographic parameters | ||||
| Mitral effective regurgitant orifice area by PISA, continuous | 4.56 (0.54-8.46) | .165 | 2.42 (0.25-12.73) | .443 |
| Mitral annular calcification | 1.37 (0.57-3.28) | .479 | 1.71 (0.64-4.58) | .287 |
| Medial-lateral mitral annular diameter ≥ 32.2mm | 1.83 (0.73-4.59) | .196 | 2.94 (1.00-5.64) | .051 |
| Nonmitral valve-related baseline echocardiographic parameters | ||||
| Left ventricular ejection fraction ≤ 56% | 1.57 (0.67-3.72) | .302 | 1.79 (0.63-5.09) | .275 |
| Left ventricular end-systolic diameter index ≥ 2.1 cm/m2* | 2.25 (1.09-4.60) | .025 | 2.44 (1.09-4.68) | .022 |
| Left ventricular end-diastolic diameter, continuous | 1.25 (0.75-2.07) | .393 | 1.12 (0.65-1.92) | .693 |
| Tricuspid annular plane systolic excursion> 18mm | 1.93 (0.79-4.71) | .147 | 2.24 (0.75-6.64) | .148 |
| Speckle-tracking | ||||
| Left ventricular global longitudinal strain less negative than −12.3% | NA | NA | 1.10 (0.38-3.25) | .857 |
95%CI, 95% confidence interval; HR, hazard ratio; NA, not applicable; PISA, proximal isovelocity surface area.
All-cause mortality and heart failure hospitalization. Increased baseline LVESDi was associated with a higher 1-year cumulative incidence of the composite of all-cause mortality or heart failure hospitalization (A) and of its separate components (B,C). Increased mid-diastolic M-L MAD was associated with earlier heart failure hospitalization (D). LVESDi, left ventricular end-systolic diameter index; M-L, medial-lateral; MAD, mitral annular diameter; TEER, transcatheter edge-to-edge repair.
Apart from LVESD, an increased LAVi (as a continuous variable) at baseline was also associated with a higher risk of all-cause mortality (HR, 1.02; 95%CI, 1.01-1.04; P=.012), and a mid-diastolic M-L MAD of ≥ 32.2mm, - with a higher risk (HR, 4.29; 95%CI, 1.55-6.90; P=.005) and earlier occurrence of HF readmissions. An above-mild MAC, while leading only to a trend toward a higher HF hospitalization risk, was nevertheless linked to excess HF hospitalizations (n=9/53, 17.0% vs n=28/357, 7.8%, P=0.040), most of which (n=6/9) were attributed to noncardiac causes.
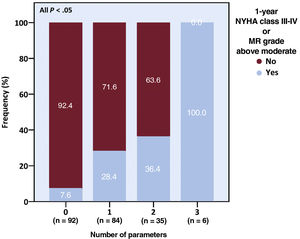
Regarding functional status and residual MR, an LAVi of ≥ 60mL/m2, an above-mild MAC, and an above-moderate TR prior to TEER were associated with a higher odds of NYHA class III-IV or above-moderate MR persistence at 1-year postprocedure, and patients exhibiting a higher number of such characteristics had higher rates of the combined outcome (table 7 of the supplementary data and figure 3). All TTE parameters associated with the 1-year endpoints, except LAVi and M-L MAD, as well as an indexed mid-diastolic M-L MAD of ≥ 20.2mm/m2 and a mitral effective regurgitant orifice area (EROA) of ≥ 0.40cm2, conferred a higher risk of greater-than-mild MR at 1 month (table 8 of the supplementary data). Of note, patients with above-mild MR at 1 month experienced a steeper intraprocedural rise in the TMPG (2 [IQR, 1-3] vs 1 [IQR, 0-2] mmHg, P=.028), were treated by fewer clips (1 [IQR, 0-1] vs 2 [IQR, 1-2]; P=.437), and were marginally more likely to exhibit above-mild MR immediately after clip deployment (n=12/24, 50% vs n=30/91, 33.0%, P=.123) if they had above-mild (compared with up-to-mild) MAC at baseline.
Functional status and grade of mitral regurgitation. A higher baseline parameter burden was associated with increased rates of significant functional impairment or MR at 1 year. The parameters were left atrial volume index of ≥ 60mL/m2, above-mild mitral annular calcification, and greater-than-moderate tricuspid regurgitation. MR, mitral regurgitation; NYHA, New York Heart Association.
Lastly, exploratory regression models that integrated baseline clinical variables and procedural aspects whose frequencies differed between the low and high LVESDi groups, year of intervention, device generation, and data availability largely reproduced the results of the TTE-only based analyses (tables 9-15 of the supplementary data). Additionally, they suggested a possible association between an increased mid-diastolic M-L MAD and both the primary outcome and all-cause mortality.
DISCUSSIONOur study evaluated the prognostic significance of TTE findings prior to TEER for chronic primary MR. Aiming for simplicity and applicability, we analyzed well-standardized, widely-accepted parameters that can be readily used in daily clinical practice. Regarding MAD, the mid-diastolic rather than the end-diastolic value was considered because of a clearer delineation of the annular edges at maximal valve opening.
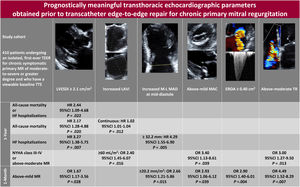
The main findings of this study were as follows (figure 4): a) an LVESDi of ≥ 2.1cm/m2 was associated with higher rate, cumulative incidence and risk of the primary outcome of all-cause mortality or HF hospitalization at 1 year and of each of its components; b) a higher LAVi (as a continuous variable) and a mid-diastolic M-L MAD of ≥ 32.2mm conferred an increased risk of all-cause mortality and HF hospitalizations, respectively; c) an LAVi of ≥ 60mL/m2, an above-mild MAC, and an above-moderate TR were associated with higher odds of NYHA class III-IV or ≥ moderate-to-severe MR persistence at 1 year; d) all the above-mentioned TTE parameters except LAVi and M-L MAD, as well as an indexed mid-diastolic M-L MAD of ≥ 20.2mm/m2 and a mitral EROA of ≥ 0.40cm2, were associated with an elevated odds of exhibiting above-mild MR at 1 month; and e) other structural, functional, and hemodynamic parameters, including LVGLS, were not predictive of any of the examined outcomes.
Central illustration. Among 410 patients undergoing mitral TEER for chronic symptomatic primary MR, preprocedural TTE findings of increased indexed left heart chambers dimensions—most importantly LVESDi—as well as mid-diastolic M-L MAD, MAC, TR extent, and mitral EROA were associated with adverse outcomes. Cutoff values represent the medians (LAVi, M-L MAD, and EROA) or 4th quartiles (LVESDi, M-L MAD index) of the cohort. Empty cells denote the absence of prognostic significance.
95%CI, 95% confidence interval; EROA, effective regurgitant orifice area; HF, heart failure; HR, hazard ratio; LAVi, left atrial volume index; LVESDi, left ventricular end-systolic diameter index; M-L, medial-lateral; MAC, mitral annular calcification; MAD, mitral annular diameter; MR, mitral regurgitation; NYHA, New York Heart Association; OR, odds ratio; TEER, transcatheter edge-to-edge repair; TR, tricuspid regurgitation; TTE, transthoracic echocardiogram.
Highlighting baseline TTE-derived measures of left cardiac chambers and MAD, along with MAC and TR, as potential markers of postinterventional clinical outcomes, our results could have been driven by the extent of the underlying disease and/or accompanying comorbidities associated with these parameters, as well as their impact on procedural success. As shown, patients with vs without an LVESDi of ≥ 2.1cm/m2 displayed a higher serum B-type natriuretic peptide level, more pronounced biventricular dysfunction, and an increased LV mass index, all of which is consistent with a more advanced myocardial remodeling process that may impair the therapeutic ability of TEER in the setting of intrinsic MR.27 Accordingly, patients with an LVESDi of ≥ 2.1cm/m2 required more clips per procedure and showed greater residual MR at 1-month and worse outcomes at 1 year. As possible manifestations of a transition toward a myopathic disorder less amenable to an isolated valvular intervention,28,29 higher LAVi and MAD were also linked to a less favorable course. As for significant MAC and TR, their association with adverse events may have been mediated by a greater burden of comorbidities,30 direct hazard,31,32 and suboptimal technical results, the latter being exemplified by the association between an above-mild MAC and an exaggerated TMPG increase, fewer deployed clips per patient, and greater residual MR.
Our study has 2 practical implications. The first is that LVESDi is more effective than LVESD in the preprocedural risk stratification of patients undergoing TEER for chronic PMR. As stressed, LVESDi correlated with procedural results and clinical events, whereas LVESD did not. Increased LVESDi may represent an earlier stage of cardiac deterioration complicating volume overload states, which may not be fully appreciated by the non-indexed, heart size variability indifferent LVESD. Indeed, most (n=62/80, 77.5%) of the patients with a high LVESD also had a high LVESDi, whereas only a few patients (n=41/330, 12.4%) within the low LVESD subgroup exhibited elevated LVESDi. Nevertheless, the association between LVESDi and the primary outcome was independent of LVESD. In addition to its better sensitivity, LVESDi may provide a more accurate estimate of cardiac remodeling in patients with lower body surface area, and specifically women, who may theoretically display normal LVESD despite advanced MR. This apparent predictive advantage of an indexed measure of dimension resembles the one observed in the aorta33 and may be attested, both conceptually and for the exact cutoff value, by future prospective studies.
The second implication arising from our work is that most echocardiographic parameters suggested by current practice guidelines and risk stratification tools as indications, contraindications, or outcome predictors for chronic PMR interventions may have limited ability to predict the clinical course following an isolated, first-time TEER performed exclusively for chronic symptomatic PMR. This could be due to the different populations studied and analytical approaches used. In this regard, LVEF of ≤ 60% and LVESD of ≥ 4.0cm, both of which indicate LV dysfunction justifying invasive treatment according to the guidelines, have been analyzed in asymptomatic individuals scheduled for either conservative34 or surgical management.35 Our patients, on the other hand, were all symptomatic, percutaneous candidates. Similarly, various indices and cutoff values of LV function17. and dimensions,7,11,14 as well as PASP18 and LVGLS,36 previously shown to predict outcomes after mitral TEER, have been examined in functional or heterogenous MR cohorts, some of which were exposed to concomitant nonmitral interventions able to influence subsequent outcomes independently. Furthermore, the prognostic usefulness of these parameters was based on mixed imaging/clinical models potentially prone to bias. By contrast, our analyses specifically included chronic PMR patients who underwent a stand-alone TEER and comprehensively focused on TTE variables.
We believe that our study could assist clinicians in the preprocedural risk stratification and postprocedural monitoring strategy used in mitral TEER for chronic PMR. Specifically, timing the intervention and tailoring the follow-up based on the presence of prognostically meaningful echocardiographic characteristics at baseline may improve outcomes and reduce futility, eventually enhancing resource utilization. Given the observational approach and paucity of data on symptom duration in the current study, implementing its findings in real-world practice may best await validation by further, prospective explorations targeting case selection and surveillance.
LimitationsFirst, the single-center, retrospective design of the study and its lack of central adjudication may hamper the generalizability of results. However, our sample was relatively large, resembled a recently published American nationwide registry,37 and was assessed by experienced echocardiologists blinded to patient files, all of which potentially improved validity. Second, the lack of follow-up data on functional status and MR grade, as well as the low absolute number of outcome events, negatively impacted statistical power, making some analyses, and particularly those that included non-TTE variables, exploratory. Nevertheless, data availability was comparable to that of previous real-world registries12,13 and was similarly distributed in the various subgroups, somewhat limiting the probability of bias. In addition, the lack of follow-up data did not affect the regression models, suggesting that it did not influence the results. Furthermore, the better-documented 1-month MR grade, which has previously been associated with longer-term outcomes38 and is currently used to determine procedural success,20 correlated well with its 1-year counterpart and was associated with most of the prognostically meaningful TTE observations, thus supporting the latter's significance. Third, our study focused on 2D TTE measurements while disregarding 3D parameters,39 as the latter could be analyzed only in a fraction of patients (n=80, 19.5%). Notwithstanding possible inaccuracies in measurement, reliability and consistency were acceptable, as indicated by good interobserver/modality agreement. Further reinforcing validity were the multivariable analyses, which are among the most comprehensive to date. Fourth, baseline medical therapy was somewhat suboptimal, precluding the extrapolation of our findings to medically optimized populations. However, this represented patient tolerance and was consistent with the real-world setting of the study.40 Last, our observations may be less applicable to patients treated with the newest-generation devices and delivery systems or to rheumatic MR cases, as these were either underrepresented or not included in the study.
CONCLUSIONSPreprocedural TTE findings of enlarged MAD, significant MAC and TR, elevated mitral EROA and, most importantly, increased indexed left heart chamber size, mainly LVESDi, are associated with a less favorable course following TEER for chronic PMR. Pending prospective validation, incorporating these variables in the clinical decision pathways preceding and following the procedure may prove beneficial.
FUNDINGNone declared.
ETHICAL CONSIDERATIONSThe study conformed to the Declaration of Helsinki and was approved by the Cedars-Sinai Institutional Review Board, which waived the need for informed consent. We confirm that possible sex/gender biases were taken into account in the preparation of this article.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCEThe study did not involve the use of artificial intelligence.
AUTHORS’ CONTRIBUTIONSA. Shechter conceptualized the project, gathered data, performed analyses, and written the first draft of the manuscript. All coauthors have participated in the revision of the text.
Preprocedural TTE is widely used to screen mitral TEER candidates. Its prognostic usefulness in the setting of chronic primary MR is not well-established.
WHAT DOES THIS STUDY ADD?In our single-center analysis of 410 patients, increased indexed left chamber size, EROA, MAD, and the extent of MAC and TR at baseline were associated with a less favorable course following the procedure. Of these, LVESDi ≥ 2.1cm/m2 was the only parameter to independently confer a higher risk of the composite of death or HF hospitalization at 1 year. In contrast, biventricular function, nonindexed heart dimensions, and PA pressure—all of which play a central role in current practice guidelines and risk models—were not predictive of outcomes.
R.R. Makkar received grant support from Edwards Lifesciences Corporation, is a consultant for Abbott Vascular, Cordis, and Medtronic, and holds equity in Entourage Medical. T. Chakravarty is a consultant, proctor, and speaker for Edwards Lifesciences and Medtronic, a consultant for Abbott Lifesciences, and is a consultant and speaker for Boston Scientific. A. Schechter was granted a general research scholarship by the California Chapter of the American College of Cardiology through the Save a Heart Foundation. The remaining authors have no conflicts of interest to disclose.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2023.12.001