Patients with combined heart failure (HF) and chronic kidney disease (CKD) have been underrepresented in clinical trials. The prevalence of CKD in these patients and their clinical profile require constant evaluation. This study aimed to analyze the prevalence of CKD, its clinical profile, and patterns of use of evidence-based medical therapies in HF across CKD stages in a contemporary cohort of ambulatory patients with HF.
MethodsFrom October 2021 to February 2022, the CARDIOREN registry included 1107 ambulatory HF patients from 13 HF clinics in Spain.
ResultsThe median age was 75 years, 63% were male, and 48% had heart failure with reduced left ventricular ejection fraction (HFrEF). A total of 654 (59.1%) had an estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2, and 122 (11%) patients with eGFR ≥ 60 mL/min/1.73 m2 had a urine albumin-creatinin ratio ≥ 30 mg/g. The most important variables associated with lower eGFR were age (R2=61%) and furosemide dose (R2=21%). The proportion of patients receiving an angiotensin-converting enzyme inhibitor (ACEI)/ angiotensin II receptor blockers (ARB), an angiotensin receptor-neprilysin inhibitor (ARNi), a sodium-glucose cotransporter 2 inhibitor (SGLT2i), or a mineralocorticoid receptor antagonist (MRA) progressively decreased with lower eGFR categories. Notably, 32% of the patients with HFrEF and an eGFR <30 mL/min/1.73 m2 received the combination of ACEI/ARB/ARNi+beta-blockers+MRA+SGLT2i.
ConclusionsIn this contemporary HF registry, 70% of patients had kidney disease. Although this population is less likely to receive evidence-based therapies, structured and specialized follow-up approaches within HF clinics may facilitate the adoption of these life-saving drugs.
Keywords
Chronic kidney disease (CKD) is a common and relevant comorbidity in patients with heart failure (HF).1–4 Population aging and the continuous improvement in diagnosis and treatment modalities are leading to increased morbidity among HF patients.5 Indeed, real-world data have shown a progressive increase in CKD over the last decades.6 For instance, a systematic analysis of the Global Burden of Disease Study 2017 estimated a global all-age CKD prevalence increase of 29% from 1990 to 2017.6 Moreover, CKD is expected to become the fifth global cause of death by 2040.7
To date, the reported prevalence of CKD in HF patients varies between 26% and 57%.1–4 However, most studies have focused on acute HF and selected populations, and albuminuria has rarely been assessed. Additionally, most information comes from patients enrolled more than 5 years ago,1–4 and therefore current data on CKD in chronic HF are scarce.
In addition, patients with advanced CKD have traditionally been excluded from clinical trials in HF, and management strategies have been largely empirical. Indeed, HF patients with concomitant CKD are often undertreated with life-prolonging guideline-recommended medication.8–10
Therefore, this study aimed: a) to evaluate the prevalence of kidney disease in a contemporary cohort of patients with chronic HF, b) to define the clinical profile, and c) to describe the patterns of use of evidence-based medical therapies in HF across CKD stages.
METHODSStudy design and populationWe prospectively evaluated a consecutive cohort of patients who attended a routine follow-up visit in Spanish HF clinics at 13 tertiary hospitals, regardless of baseline estimated glomerular filtration rate (eGFR) from October 2021 to February 2022. Diagnosis of HF was performed according to current European guidelines.11 The only exclusion criterion was refusal to participate.
Data were collected on patient demographics, medical history, medical and device therapy at baseline, vital signs, and physical examination. Clinical congestion was assessed by the composite congestion score (CCS), which included orthopnea (0-3), leg edema (0-3), and jugular engorgement (0-3).12 All the variables were previously specified and communicated to the research team, who had training meetings prior to the start of the study. Data on medical treatment were obtained directly from the patient's history and was verified with the electronic prescription data.
This study complied with the Declaration of Helsinki and was approved by the ethics committees of participating centers. Informed consent was obtained from all patients.
Laboratory analysisBlood and urine tests were assessed at baseline (within a 48-hour window from inclusion) and were analyzed in the local laboratory at each center. eGFR was calculated based on creatinine levels using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation and stratified according to KDIGO 2012 classification into 4 clinical strata: <30 mL/min/1.73 m2 (G4-G5); 30-44 mL/min/1.73 m2 (G3b), 45-59 mL/min/1.73 m2 (G3a), or ≥ 60 mL/min/1.73 m2.13 All patients had a prior eGFR assessment available in their medical chart for eGFR confirmation. The urine albumin-creatinine ratio was assessed in the first-morning urine sample, and albuminuria was stratified into 3 categories: A1 (normal to mildly increased): <30mg/g, A2 (moderately increased): 30-300mg/g, and A3 (severely increased):> 300mg/g.
Statistical analysisContinuous variables are presented as median [interquartile range (IQR)]. Categorical variables are expressed as percentages. Comparisons across eGFR categories were performed by the chi-square test for categorical variables. For continuous variables, 1 -way analysis of variance (ANOVA) and the Kruskal-Wallis test were used for variables with normal and nonnormal distribution, respectively. The variables associated with eGFR were evaluated by multivariate linear regression analysis. The contribution of the exposures to the proportion of the dependent variable variation was evaluated by R2. We simultaneously tested the linearity assumption for all continuous variables, and the variables were transformed with fractional polynomials when appropriate. Next, we derived a reduced and parsimonious model using backward step-down selection on prior knowledge/biological plausibility, independent of the P-value. We set a 2 -sided P <.05 as the threshold for statistical significance. The covariates included in the final model were age, sex, hypertension, smoking, chronic obstructive pulmonary disease (COPD), ictus, dementia, baseline baselinediastolic blood pressure, left ventricular ejection fraction (LVEF), CA125 levels, renin-angiotensin system inhibitor (RASi), sodium-glucose cotransporter inhibitors (SGLT2i), mineralcorticoid receptor antagonists (MRA), beta-blockers, and baseline baselinefurosemide dose. Multinomial logistic regression analysis was performed to evaluate the association between HF with reduced ejection fraction (HFrEF) therapies according to eGFR. This model was adjusted for age, sex, tobacco use, hypertension, dyslipidemia, diabetes mellitus, ischemic cardiomyopathy, valvular disease, atrial fibrillation, COPD, ictus, cancer, CKD, dementia, heart rate, and systolic and diastolic blood pressure. Stata 15.1 (Stata Statistical Software, Release 15, 2017; StataCorp LP, United States) was used for these analyses.
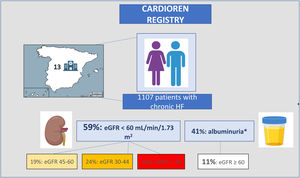
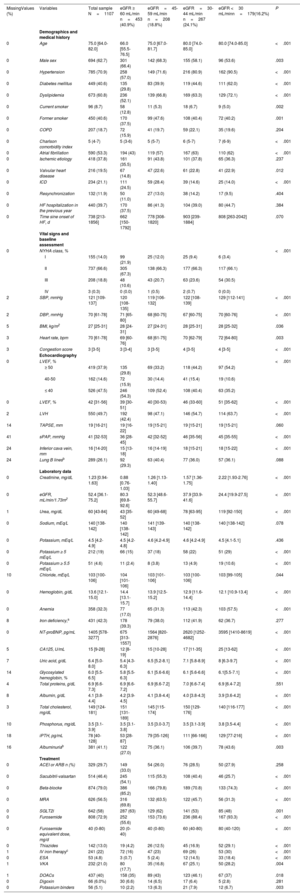
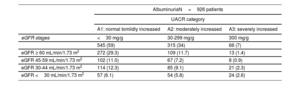
RESULTSA total of 1107 patients were included (figure 1). The median number of patients from each hospital was 100 per center (figure 1 of the supplementary data). The total cohort's median age was 75 [IQR: 64-82] years, 694 (62.7%) were male, and 688 (62.1%) had reduced or mildly reduced left ventricular ejection fraction (LVEF <50%) (table 1). The median [IQR] creatinine and eGFR were 1.23mg/dL [0.94-1.63] and 52.4 mL/min/1.73 m2 [36.1-75.2], respectively. The median Ntprobnp and CA125 were 1405 pg/mL [578-3277] and 15 U/mL [9-28], respectively. A total of 654 patients (59.1%) had an estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 (figure 1). Among those in which albuminuria was measured (926/1107), 381 (41%) had a urine albumin-creatinine ratio (UACR) ≥ 30 mg/g (table 2).
Baseline characteristics
| MissingValues (%) | Variables | Total sample N=1107 | eGFR ≥ 60 mL/min n=453 (40.9%) | eGFR=45-59 mL/min n=208 (18.8%) | eGFR=30-44 mL/min n=267 (24.1%) | eGFR <30 mL/minn=179(16.2%) | P |
|---|---|---|---|---|---|---|---|
| Demographics and medical history | |||||||
| 0 | Age | 75.0 [64.0-82.0] | 66.0 [55.5-76.5] | 75.0 [67.0-81.7] | 80.0 [74.0-85.0] | 80.0 [74.0-85.0] | <.001 |
| 0 | Male sex | 694 (62.7) | 301 (66.4) | 142 (68.3) | 155 (58.1) | 96 (53.6) | .003 |
| 0 | Hypertension | 785 (70.9) | 258 (57.0) | 149 (71.6) | 216 (80.9) | 162 (90.5) | <.001 |
| 0 | Diabetes mellitus | 449 (40.6) | 135 (29.8) | 83 (39.9) | 119 (44.6) | 111 (62.0) | <.001 |
| 0 | Dyslipidemia | 673 (60.8) | 236 (52.1) | 139 (66.8) | 169 (63.3) | 129 (72.1) | <.001 |
| 0 | Current smoker | 96 (8.7) | 58 (12.8) | 11 (5.3) | 18 (6.7) | 9 (5.0) | .002 |
| 0 | Former smoker | 450 (40.6) | 170 (37.5) | 99 (47.6) | 108 (40.4) | 72 (40.2) | .001 |
| 0 | COPD | 207 (18.7) | 72 (15.9) | 41 (19.7) | 59 (22.1) | 35 (19.6) | .204 |
| 0 | Charlson comorbidity index | 5 (4-7) | 5 (3-6) | 5 (5-7) | 6 (5-7) | 7 (6-9) | <.001 |
| 0 | Atrial fibrillation | 590 (53.3) | 194 (43) | 119 (57) | 167 (63) | 110 (62) | <.001 |
| 0 | Ischemic etiology | 418 (37.8) | 161 (35.5) | 91 (43.8) | 101 (37.8) | 65 (36.3) | .237 |
| 0 | Valvular heart disease | 216 (19.5) | 67 (14.8) | 47 (22.6) | 61 (22.8) | 41 (22.9) | .012 |
| 0 | ICD | 234 (21.1) | 111 (24.5) | 59 (28.4) | 39 (14.6) | 25 (14.0) | <.001 |
| 0 | Resynchronization | 132 (11.9) | 50 (11.0) | 27 (13.0) | 38 (14.2) | 17 (9.5) | .404 |
| 0 | HF hospitalization in the previous year | 440 (39.7) | 170 (37.5) | 86 (41.3) | 104 (39.0) | 80 (44.7) | .384 |
| 0 | Time sine onset of HF, d | 738 [213-1856] | 662 [150-1792] | 778 [308-1820] | 903 [239-1884] | 808 [263-2042] | .070 |
| Vital signs and baseline assessment | |||||||
| 0 | NYHA class, % | <.001 | |||||
| I | 155 (14.0) | 99 (21.9) | 25 (12.0) | 25 (9.4) | 6 (3.4) | ||
| II | 737 (66.6) | 305 (67.3) | 138 (66.3) | 177 (66.3) | 117 (66.1) | ||
| III | 208 (18.8) | 48 (10.6) | 43 (20.7) | 63 (23.6) | 54 (30.5) | ||
| IV | 3 (0.3) | 0 (0.0) | 1 (0.5) | 2 (0.7) | 0 (0.0) | ||
| 2 | SBP, mmHg | 121 [109-137] | 120 [108-135] | 119 [106-132] | 122 [108-139] | 129 [112-141] | <.001 |
| 2 | DBP, mmHg | 70 [61-78] | 71 [65-80] | 68 [60-75] | 67 [60-75] | 70 [60-76] | <.001 |
| 5 | BMI, kg/m2 | 27 [25-31] | 28 [24-31] | 27 [24-31] | 28 [25-31] | 28 [25-32] | .036 |
| 3 | Heart rate, bpm | 70 [61-78] | 69 [60-76] | 68 [61-75] | 70 [62-79] | 72 [64-80] | .003 |
| 3 | Congestion score | 3 [3-5] | 3 [3-4] | 3 [3-5] | 4 [3-5] | 4 [3-5] | <.001 |
| Echocardiography | |||||||
| 0 | LVEF, % | <.001 | |||||
| ≥ 50 | 419 (37.9) | 135 (29.8) | 69 (33.2) | 118 (44.2) | 97 (54.2) | ||
| 40-50 | 162 (14.6) | 72 (15.9) | 30 (14.4) | 41 (15.4) | 19 (10.6) | ||
| ≤ 40 | 526 (47.5) | 246 (54.3) | 109 (52.4) | 108 (40.4) | 63 (35.2) | ||
| 0 | LVEF, % | 42 [31-56] | 39 [30-51] | 40 [30-53) | 46 (33-60] | 51 [35-62] | <.001 |
| 2 | LVH | 550 (49.7) | 192 (42.4) | 98 (47.1) | 146 (54.7) | 114 (63.7) | <.001 |
| 14 | TAPSE, mm | 19 [16-21] | 19 [16-22] | 19 [15-21] | 19 [15-21] | 19 [15-21] | .060 |
| 41 | sPAP, mmHg | 41 [32-53] | 36 [28-45] | 42 [32-52] | 46 [35-56] | 45 [35-55] | <.001 |
| 24 | Inferior cava vein, mm | 16 [14-20] | 15 [13-18] | 16 [14-19] | 18 [15-21] | 18 [15-22] | <.001 |
| 24 | Lung B linesb | 289 (26.1) | 92 (29.3) | 63 (40.4) | 77 (36.0) | 57 (36.1) | .088 |
| Laboratory data | |||||||
| 0 | Creatinine, mg/dL | 1.23 [0.94-1.63] | 0.88 [0.76-1.03] | 1.26 [1.13-1.40] | 1.57 [1.36-1.75] | 2.22 [1.93-2.76] | <.001 |
| 0 | eGFR, mL/min/1.73m2 | 52.4 [36.1-75.2] | 80.3 [69.8-92.6] | 52.3 [48.6-55.7] | 37.9 [33.9-41.6] | 24.4 [19.9-27.5] | <.001 |
| 1 | Urea, mg/dL | 60 [43-84] | 43 [35-52] | 60 [49-68] | 78 [63-95] | 119 [92-150] | <.001 |
| 0 | Sodium, mEq/L | 140 [138-142] | 140 [138-142] | 141 [139-143] | 140 [138-142] | 140 [138-142] | .078 |
| 0 | Potassium, mEq/L | 4.5 [4.2-4.9] | 4.5 [4.2-4.8] | 4.6 [4.2-4.9] | 4.6 [4.2-4.9] | 4.5 [4.1-5.1] | .436 |
| 0 | Potassium ≥ 5 mEq/L | 212 (19) | 66 (15) | 37 (18) | 58 (22) | 51 (29) | <.001 |
| 0 | Potassium ≥ 5.5 mEq/L | 51 (4.6) | 11 (2.4) | 8 (3.8) | 13 (4.9) | 19 (10.6) | <.001 |
| 10 | Chloride, mEq/L | 103 [100-106] | 104 [101-106] | 103 [101-106] | 103 [100-106] | 103 [99-105] | .044 |
| 0 | Hemoglobin, g/dL | 13.6 [12.1-15.0] | 14.4 [13.1-15.7] | 13.9 [12.5-15.2] | 12.9 [11.6-14.4] | 12.1 [10.9-13.4] | <.001 |
| 0 | Anemia | 358 (32.3) | 77 (17.0) | 65 (31.3) | 113 (42.3) | 103 (57.5) | <.001 |
| 8 | Iron deficiency,a | 431 (42.3) | 178 (39.3) | 79 (38.0) | 112 (41.9) | 62 (36.7) | .277 |
| 0 | NT-proBNP, pg/mL | 1405 [578-3277] | 675 [313-1557] | 1564 [820-2876] | 2620 [1252-4682] | 3595 [1410-8619] | <.001 |
| 5 | CA125, U/mL | 15 [9-28] | 12 [8-19] | 15 [10-28] | 17 [11-35] | 25 [13-62] | <.001 |
| 7 | Uric acid, g/dL | 6.4 [5.0-8.0] | 5.4 [4.3-6.3] | 6.5 [5.2-8.1] | 7.1 [5.8-8.9] | 8 [6.3-9.7] | <.001 |
| 14 | Glycosylated hemoglobin, % | 6.0 [5.5-6.5] | 5.8 [5.5-6.3] | 6.1 [5.6-6.6] | 6.1 [5.6-6.6] | 6.1[5.5-7.1] | <.001 |
| 4 | Total proteins, g/dL | 6.9 [6.6-7.3] | 6.9 [6.6-7.2] | 6.9 [6.6-7.2] | 7.0 [6.6-7.4] | 6.9 [6.4-7.2] | .551 |
| 8 | Albumin, g/dL | 4.1 [3.8-4.4] | 4.2 [3.9-4.5] | 4.1 [3.8-4.4] | 4.0 [3.8-4.3] | 3.9 [3.6-4.2] | <.001 |
| 3 | Total cholesterol, mg/dL | 149 [124-181] | 151 [131-189] | 145 [115-174] | 150 [129-176] | 140 [116-177] | <.001 |
| 10 | Phosphorus, mg/dL | 3.5 [3.1-3.9] | 3.5 [3.1-3.8] | 3.5 [3.0-3.7] | 3.5 [3.1-3.9] | 3.8 [3.5-4.4] | <.001 |
| 18 | iPTH, pg/mL | 78 [40-128] | 53 [28-87] | 79 [35-126] | 111 [66-166] | 129 [77-216] | <.001 |
| 16 | Albuminuriab | 381 (41.1) | 122 (27.0) | 75 (36.1) | 106 (39.7) | 78 (43.6) | .003 |
| Treatment | |||||||
| 0 | ACEI or ARB n (%) | 329 (29.7) | 149 (33.0) | 54 (26.0) | 76 (28.5) | 50 (27.9) | .258 |
| 0 | Sacubitril-valsartan | 514 (46.4) | 245 (54.1) | 115 (55.3) | 108 (40.4) | 46 (25.7) | <.001 |
| 0 | Beta-blocke | 874 (79.0) | 386 (85.2) | 166 (79.8) | 189 (70.8) | 133 (74.3) | <.001 |
| 0 | MRA | 626 (56.5) | 316 (69.8) | 132 (63.5) | 122 (45.7) | 56 (31.3) | <.001 |
| 0 | SGLT2i | 642 (58) | 287 (63) | 129 (62) | 141 (53) | 85 (48) | .001 |
| 0 | Furosemide | 808 (72.9) | 252 (55.6) | 153 (73.6) | 236 (88.4) | 167 (93.3) | <.001 |
| 0 | Furosemide equivalent dose, mg/d | 40 (0-80) | 20 (0-40) | 40 (0-80) | 60 (40-80) | 80 (40-120) | <.001 |
| 0 | Thiazides | 142 (13.0) | 19 (4.2) | 26 (12.5) | 45 (16.9) | 52 (29.1) | <.001 |
| 1 | IV iron therapyc | 241 (22) | 72 (16) | 47 (23) | 69 (26) | 53 (30) | <.001 |
| 0 | ESA | 53 (4.8) | 3 (0.7) | 5 (2.4) | 12 (14.5) | 33 (18.4) | <.001 |
| 1 | VKA | 232 (21.0) | 80 (17.7) | 35 (16.8) | 67 (25.1) | 50 (28.2) | .004 |
| 1 | DOACs | 437 (40) | 158 (35) | 89 (43) | 123 (46.1) | 67 (37) | .018 |
| 0 | Digoxin | 66 (6.0%) | 30 (6.6) | 14 (6.5) | 17 (6.4) | 5 (2.8) | .281 |
| 0 | Potassium binders | 56 (5.1) | 10 (2.2) | 13 (6.3) | 21 (7.9) | 12 (6.7) | .003 |
ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin II receptor blockers; BMI, body mass index; CA125, cancer antigen 125; COPD, chronic obstructive pulmonary disease; DBP, diastolic blood pressure; DOACs, direct oral anticoagulants; eGFR, estimated glomerular filtration rate; ESA, erythropoiesis-stimulating agent; HF, heart failure; ICD, implantable cardioverter-defibrillator; IV, intravenous; LVEF, left ventricular ejection fraction; LVH, left ventricular hypertrophy; MRA, mineralocorticoid receptor antagonists; NTproBNP, N-terminal pro-brain natriuretic peptide; NYHA, New York Heart Association; SBP, systolic blood pressure; SLGT2i, sodium-glucose cotransporter inhibitors; sPAP, systolic pulmonary artery pressure; TAPSE, tricuspid annular plane systolic excursion; VKA, vitamin K antagonists.
Data are expressed as No. (%), mean±standard deviation, or median [interquartile range].
UACR categories along eGFR stages
| AlbuminuriaN=926 patients | |||
|---|---|---|---|
| UACR category | |||
| A1: normal tomildly increased | A2: moderately increased | A3: severely increased | |
| eGFR stages | <30 mg/g | 30-299 mg/g | 300 mg/g |
| 545 (59) | 315 (34) | 66 (7) | |
| eGFR ≥ 60 mL/min/1.73 m2 | 272 (29.3) | 109 (11.7) | 13 (1.4) |
| eGFR 45-59 mL/min/1.73 m2 | 102 (11.0) | 67 (7.2) | 8 (0.9) |
| eGFR 30-44 mL/min/1.73 m2 | 114 (12.3) | 85 (9.1) | 21 (2.3) |
| eGFR <30 mL/min/1.73 m2 | 57 (6.1) | 54 (5.8) | 24 (2.6) |
eGFR, estimated glomerular filtration rate; UACR, urine albumin-creatinine ratio.
Data are expressed as No. (%).
Demographics, baseline characteristics, and pharmacological treatment stratified according to eGFR categories are summarized in table 1. The distribution of the sample across eGFR categories was: 453 (40.9%) G1 and G2 categories, 208 (18.8%) G3a category, 267 (24.1%) G3b category, and 179 (16.2%) G4 and G5 categories. Of note, 122 (11%) patients with eGFR ≥ 60 mL/min/1.73m2 had a UACR ≥ 30 mg/g (table 2). Overall, the baseline risk profile worsened from higher to lower eGFR. Similarly, patients with severely reduced eGFR (< 30 mL/min/1.73 m2) showed higher NTproBPN [3595 (1411-8591) vs 1213 (508-2776); P <.001] and CA125 [25 (13-63) vs 14 (9-24), P <.001)] plasma levels and were more likely to have anemia (P <.001) and hyperkalemia (serum potassium ≥ 5.5 mEq/L) (P <.001). The HF phenotype also differed across eGFR strata. LVEF progressively increased from higher to lower eGFR categories. In fact, more than half (54.2%) of the patients with severely reduced eGFR (G4 and G5 categories) had a preserved LVEF.
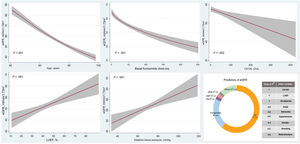
Factors associated with lower estimated glomerular filtration rateMultivariate analysis revealed that independent variables associated with lower eGFR were, in order of importance (and explaining up to 82% of the model variability): age (negative, R2: 62%, P <.001) and furosemide dose (negative, R2: 21%, P <.001). Among other covariates associated with lower eGFR, CA125 showed an inverse and linear relationship with eGFR (R2: 1%, P=.002), whereas the association was positive and linear for diastolic blood pressure and LVEF (R2: 2%, P <.001 and R2: 1%, P <.001, respectively) (figure 2). The R2 value of the model was 47%.
Predictors of eGFR after multivariate analysis. CA125, cancer antigen 125; CKD, chronic kidney disease; DBP, diastolic blood pressure; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonists; RASi, renin-angiotensin system inhibitors.
In the overall population (regardless of LVEF), the prescription of loop diuretics and thiazides progressively increased from higher to lower eGFR categories. Almost 30% of patients with an eGFR <30 mL/min/1.73 m2 were on furosemide plus thiazides at inclusion in the registry. A similar pattern was observed with furosemide equivalent doses, with higher doses in patients with lower eGFR.
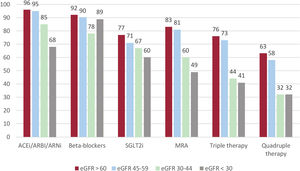
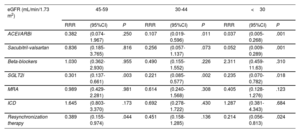
Of the entire sample, 526 patients (48%) had HFrEF (LVEF ≤ 40%). When we analyzed the prescription of guideline-directed therapy across eGFR strata among patients with HFrEF, we observed that the percentage of patients prescribed renin-angiotensin system inhibitors (RASi), angiotensin receptor-neprilysin inhibitors (ARNI), sodium-glucose cotransporter inhibitors (SGLT2i), or mineralocorticoid receptor antagonist (MRA) progressively decreased from higher to lower eGFR categories (figure 3). Among patients with eGFR ≥ 45 mL/min/1.73 m2, implementation therapy was similar, with more than 90% prescribed RASi or ARNI, 80% MRAs, and 70% SGLT2i. In patients with eGFR <45 mL/min/1.73 m2, the proportions of patients receiving these therapies were lower but was higher than 50%. When evaluating the proportions of patients on triple or quadruple therapy, we found high implementation in patients with eGFR ≥ 45 mL/min/1.73 m2 (more than 70% and 60% respectively), and moderate implementation (> 40% and> 30% respectively) in those with eGFR <45 mL/min/1.73m2. These differences persisted after adjustment for comorbidities and medication use in the multivariable analysis compared with patients with eGFR>60 mL/min/1.73 m2 (reference category) (table 3).
Percentage of treatments by estimated glomerular filtration rate (eGFR) strata among patients with heart failure with reduced ejection fraction (HFrEF). ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin II receptor blockers; ARNi, angiotensin receptor-neprilysin inhibitors; MRA, mineralocorticoid receptor antagonists; SLGT2i, sodium-glucose cotransporter inhibitors.
Medical and device treatment in HFrEF patients across categories of eGFR after multivariate adjustment
| eGFR (mL/min/1.73 m2) | 45-59 | 30-44 | <30 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| RRR | (95%CI) | P | RRR | (95%CI) | P | RRR | (95%CI) | P | |
| ACEI/ARBi | 0.382 | (0.074-1.967) | .250 | 0.107 | (0.019-0.596) | .011 | 0.037 | (0.005-0.268) | .001 |
| Sacubitril-valsartan | 0.836 | (0.185-3.765) | .816 | 0.256 | (0.057-1.137) | .073 | 0.052 | (0.009-0.289) | .001 |
| Beta-blockers | 1.030 | (0.362-2.930) | .955 | 0.490 | (0.155-1.552) | .226 | 2.311 | (0.459-11.63) | .310 |
| SGLT2i | 0.301 | (0.137-0.661) | .003 | 0.221 | (0.085-0.577) | .002 | 0.235 | (0.070-0.782) | .018 |
| MRA | 0.989 | (0.429-2.281) | .981 | 0.614 | (0.240-1.568) | .308 | 0.405 | (0.128-1.276) | .123 |
| ICD | 1.645 | (0.803-3.370) | .173 | 0.692 | (0.278-1.722) | .430 | 1.287 | (0.381-4.343) | .684 |
| Resynchronization therapy | 0.389 | (0.155-0.974) | .044 | 0.451 | (0.158-1.285) | .136 | 0.214 | (0.056-0.813) | .024 |
ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin II receptor blockers; CI, confidence interval; eGFR, estimated glomerular filtration rate; HFrEF, heart failure with reduced ejection fraction; ICD, implantable cardioverter-defibrillator; MRA, mineralocorticoid receptor antagonists; RRR, relative risk reduction; SLGT2i, sodium-glucose cotransporter inhibitors.
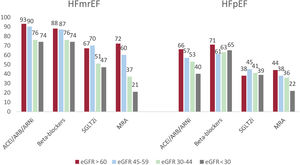
On the other hand, 419 patients (37.9%) and 162 patients (14.6%) had HFpEF and HF with mildly reduced ejection fraction, respectively. Patterns of use of guideline-directed medical therapies in patients with HFmrEF were closer to HFrEF patients, whereas lower rates were noted in patients with HFpEF (figure 4).
Percentage of treatments by estimated glomerular filtration rate (eGFR) strata among patients with heart failure with mildly reduced ejection fraction (HFmrEF) and preserved ejection fraction (HFpEF). ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin II receptor blockers; ARNi, angiotensin receptor-neprilysin inhibitors; MRA, mineralocorticoid receptor antagonists; SLGT2i, sodium-glucose cotransporter inhibitors.
We identified 3 major findings in this contemporary, multicenter, and prospective cohort evaluating more than 1000 patients with chronic HF in Spain. First, to the best of our knowledge, the prevalence of kidney disease is the highest reported in a stable HF population,1–4 including one of the most extensive series of albuminuria in chronic HF patients. Second, the prospective design allowed us to describe the current clinical profile and management of a large population with chronic HF across all eGFR categories. Finally, this is the first study to assess the implementation of quadruple therapy in a broad population of patients with stable HFrEF and kidney disease.
HF and CKD frequently coexist due to overlapping pathophysiology (ie, cardiorenal syndrome) or shared cardiometabolic risk factors that drive both disease states in parallel. However, despite the increasing awareness of CKD as a risk multiplier comorbidity in HF, contemporary real-life reports depicting the clinical profile of patients with combined chronic HF and CKD are scarce.14 Furthermore, there are few publications with information on albuminuria, congestion status, and kidney-specific comorbidities such as anemia, iron deficiency, disturbances of calcium-phosphate metabolism, and hyperkalemia.
In the present cohort, up to 70% of chronic patients with HF had some degree of kidney dysfunction. This remarkably high prevalence may be the result of several factors. First, this study included contemporary real-life patients characterized by older age, an increased prevalence of HFpEF, and a high burden of risk factors and comorbidities. In addition, albuminuria measurement (available in 83% of the patients) identified kidney impairment in 11% of patients with eGFR ≥60 mL/min/1.73 m2. Finally, although the inclusion of patients with HF in specific follow-up programs is currently widespread, a selection bias might have contributed to this high prevalence.
Interestingly, and despite the high prevalence of CKD in this population, the clinical profile of HF differed across eGFR categories. Patients with higher eGFR were predominantly male, with a lower comorbidity burden and reduced LVEF. In contrast, the clinical phenotype of patients with combined HF and advanced CKD was mainly characterized by advanced age, female sex, and preserved LVEF. Furthermore, impaired volume homeostasis was a prominent presenting feature of this cardiorenal phenotype. This study shows that patients with advanced CKD, especially those with eGFR <45 mL/min/m2, had more evidence of volume overload as assessed by CCS, biomarkers (CA125), and ultrasound surrogates of venous congestion. Interestingly, after multivariable adjustment, CA125 (and not NTproBNP) was the only surrogate marker of congestion associated with lower eGFR. An explanation for this finding could be that CA125 values are not influenced by LVEF, age, or eGFR and are highly correlated to proxies of right-sided HF (all commonly observed in this population).14,15 CA125, as a marker of fluid overload, may be helpful to identify these patients at higher risk of congestive nephropathy. Indeed, considering the demonstrated association between CA125 and renal congestion,16,17 it is quite likely that congestive nephropathy is partly to blame for CKD progression. This aspect might also explain the inverse association between furosemide equivalent doses and eGFR (higher doses in patients with more severe congestion status).
Another relevant aspect that deserves to be highlighted is the high prevalence of kidney-related comorbidities as eGFR declined. For instance, we observed a stepwise increase in the prevalence of anemia from higher to lower eGFR categories. Of note, this finding was unconnected to iron status since we observed no differences in iron deficiency across eGFR categories. Therefore, this was probably kidney-related anemia, a common and frequently overlooked comorbidity in patients with HF.
Guideline-recommended therapy in patients with heart failure with reduced ejection fraction and chronic kidney diseaseGuideline-directed medical therapy has been shown to reduce morbidity and mortality in patients with HFrEF and is recommended as a class I indication in clinical practice guidelines for patients with HFrEF.11 However, patients with advanced CKD have been traditionally excluded from cardiovascular clinical trials. Since these drugs may induce an initial eGFR decline, clinicians often struggle to initiate or up-titrate these therapies, as any deterioration in kidney function is often perceived as deleterious.18–20 Indeed, the presence of kidney disease is one of the main reasons for ineffective drug implementation in HF patients, even in patients with mild to moderate CKD.8,10
This trend was also observed in the present cohort, in which the proportion of patients receiving guideline-directed medical therapy (GDMT) decreased in parallel to the eGFR decline. Nonetheless, it is important to highlight that the implementation of GDMT across eGFR strata is among the highest reported in the literature. For instance, the Swedish HF registry has recently published the prescription of guideline-recommended therapies in 31 668 patients with HF, according to eGFR.8 In this registry, the proportions receiving triple therapy (combination of RASi/ARNI+MRA+beta-blockers) were 38%, 35%, 28%, and 15% for eGFR ≥ 60, 45-59, 30-44, and <30 mL/min/1.73 m2, respectively. Although the baseline characteristics were slightly different, these implementation rates are far from the proportions of GDMT achieved in our study, in which the patients receiving triple therapy were significantly higher, even in those with advanced CKD. Finally, the proportion of devices is higher than that reported in other registries or contemporaneous HFrEF trials such as the DAPA HF trial, in which the use of ICDs was 26%.21
On the other hand, the 2021 European Society of Cardiology guidelines included treatment recommendations for patients with HF and mildly reduced LVEF (class IIb recommendations),11 whereas no specific treatment for HFpEF was updated. We describe prescriptions patterns in both the HFmrEF and HFpEF populations. Implementation therapy was similar to that in previous reports with the use of RASi and beta-blockers.22,23 However, MRNA use was higher. In addition, about 40% of our HFpEF patients were on SGLT2i therapy, regardless of kidney function. The use of SGLT2i in this population was probably partly due to the prevalence of diabetes mellitus and the novel evidence of the benefits of this therapy in HFpEF.24.
Another possible explanation for all these differences might be that this population comes from specific HF programs, which may be a potential source of bias but also highlights the possible advantages of these structured and specialized models of care.
Future directions and clinical implicationsThe present registry reinforces the message that the prevalence of kidney disease in real-life patients with HF is remarkably high. Population longevity, the increased prevalence of cardiovascular risk factors, and associated comorbidities have probably contributed to the growth of this phenotype of cardiorenal disease, characterized by older age, a high burden of comorbidities and congestion, and preserved LVEF. Therefore, clinicians should be aware of this emerging entity and its clinical implications. Early diagnosis and multidisciplinary teamwork could lead to the early initiation of disease-modifying therapies and a more personalized and structured approach. In addition, developing specific coordinated cardiorenal management programs to improve diagnosis and offer appropriate evidence-based therapies, education, and suitable follow-up may improve outcomes in this increasingly complex population.25
LimitationsThis study has some limitations. First, although all patients had a prior eGFR assessment available in their medical chart, this measurement was not prespecified within a specific time interval. Accordingly, the measurement at the moment of inclusion may have misclassified some patients. However, this is the information commonly available in routine clinical practice. In addition, albuminuria was not determined in 17% of the patients, which could have modified the classification of CKD in some patients. Second, patients were enrolled in specialized HF clinics. Therefore, extrapolation to other follow-up approaches, countries, or health care systems needs to be performed with caution. Third, even though the registry protocol required consecutive enrolment, we cannot guarantee that this requirement was respected in all centers, and information on refusal to participate was not collected. Fourth, we acknowledge that the information on pharmacy fills may not necessarily mean that the pills are being ingested.
CONCLUSIONSOverall, 70% of patients with chronic HF had some degree of kidney dysfunction. Evaluating albuminuria improved the detection of kidney disease. Patients with concomitant HF and kidney impairment had a worse baseline profile. Although the implementation of evidence-based therapies in this population with HFrEF was high, those with concomitant kidney disease were less likely to be prescribed evidence-based medical therapies.
FUNDINGThis work was supported by AstraZeneca Spain.
AUTHORS’ CONTRIBUTIONSM. Cobo Marcos and J. Núñez contributed to the conception and design of the study. M. Cobo Marcos and R. de la Espriella are equally first authors. M. Cobo Marcos, J. Núñez, R. de la Espriella, J. Gayán Ordás, P Llàcer, A. Pomares, A. Fort, I. Ponz de Antonio, A. Méndez, Z. Blázquez-Bermejo, P. Caravaca Pérez, J. Rubio Gracia, A. Recio-Mayoral, I. Zegrí, JM. García Pinilla, E. Montero Hernández, A. Castro, M.J. Soler, J.L. Górriz, R. Bascompte Claret, P. Fluvià-Brugués and L. Manzano contributed to the analysis and interpretation of data, drafting of the manuscript and critical revision for important intellectual content, and approved the final version of the submitted manuscript.
CONFLICTS OF INTERESTAll the authors report grants from AstraZeneca during the conduct of the study. M. Cobo Marcos reports personal fees from AstraZeneca, Vifor Pharma, Novartis, Rovi, Boehringher-Ingelheim, Novonordisk, Esteve, and Bayer. A. Pomares reports personal fees from Pfizer and support for attending meetings from Novartis and Bayer. A. Méndez reports personal fees from AstraZeneca and Novartis, support for attending meetings from Novartis, Boehringher-Ingelheim, and AstraZeneca; J. Rubio Gracia reports personal fees from AstraZeneca, Novartis, Boehringher-Ingelheim, Esteve, and Bayer. A. Recio-Mayoral reports personal fees from AstraZeneca, Novartis, Boehringher-Ingelheim, Janssen, Bayer, MSD and Vifor Pharma, and support for attending meetings from Bayer, MSD and Novartis. J.M. Garía Pinilla reports personal fees from AstraZeneca, Vifor Pharma, Novartis, Boehringher-Ingelheim, Pfizer, and Bayer. E. Montero Hernández reports personal speaker fees from AstraZeneca, Boehringher-Ingelheim and Lilly. M.J. Soler Romeo reports personal fees from NovoNordisk, Jansen, Boehringer-Ingelheim, Mundipharma, Esteve, Fresenius, Ingelheim Lilly, Vifor, ICU Medical, Travere Therapeutics, grants from Boehringher-Ingelheim, Mataró TV3 and Instituto Carlos III; Editor-in-Chief of CKJ; M.J. Soler Romeo has a patent 202131356 issued. J.L. Górriz reports research grant support from AstraZeneca, CSL Vifor, personal speaker fees from AstraZeneca, Boehringher-Ingelheim, Lilly, Novartis, and Novonordisk. P. Fluvià-Brugués reports honoraria for lectures from Novartis, AstraZeneca, Boehringer-Ingelheim and Rovi and support from attending meetings from Novartis, AstraZeneca and Boehringer-Ingelheim. L. Manzano reports honoraria for lectures from AstraZeneca, Novartis, Boehringher-Ingelheim, Rovi and Bayer and support for attending meetings from AstraZeneca, Pfizer, Novartis, Boehringher-Ingelheim, and participation on advisory boards from Bayer, Lilly, Novartis, and Pfizer. J. Núñez reports grants from Vifor Pharma, personal fees from Novartis, Rovi, Boehringher-Ingelheim, Novonordisk, and Bayer. All other authors declare no competing interests.
- -
Chronic kidney disease is a common and important comorbidity in patients with heart failure.
- -
The cardiorenal nexus encompasses a bidirectional relationship that worsens prognosis and may complicate pharmacological management in patients with concomitant heart and kidney disease.
- -
Patients with advanced chronic kidney disease have traditionally been excluded from clinical trials in HF, and management strategies have been largely empirical.
- -
The prevalence of kidney disease, 70%, is the highest reported in a stable HF population.
- -
We describe the cardiorenal phenotype, characterized by advanced age, female sex, impaired volume homeostasis, and preserved left ventricular ejection fraction.
- -
This is the first study to assess the implementation of quadruple therapy in a broad population of patients with stable heart failure with reduced ejection fraction and kidney disease.
- -
The implementation of guideline-directed medical therapy across estimated glomerula filtration rate strata is among the highest reported in the literature, highlighting the possible advantages of structured and specialized models of care.