Few data exist on the outcomes of valvular cardiomyopathy patients referred for defibrillator implantation for primary prevention. The aim of the present study was to describe the outcomes of this cardiomyopathy subgroup.
MethodsThis multicenter retrospective study included consecutive patients referred for defibrillator implantation to 15 Spanish centers in 2010 and 2011, and to 3 centers after 1 January 2008.
ResultsOf 1174 patients, 73 (6.2%) had valvular cardiomyopathy. These patients had worse functional class, wider QRS, and a history of atrial fibrillation vs patients with ischemic (n=659; 56.1%) or dilated (n=442; 37.6%) cardiomyopathy. During a follow-up of 38.1 ± 21.3 months, 197 patients (16.7%) died, without significant differences among the groups (19.2% in the valvular cardiomyopathy group, 15.8% in the ischemic cardiomyopathy group, and 17.9% in the dilated cardiomyopathy group; P=.2); 136 died of cardiovascular causes (11.6%), without significant differences among the groups (12.3%, 10.5%, and 13.1%, respectively; P=.1). Although there were no differences in the proportion of appropriate defibrillator interventions (13.7%, 17.9%, and 18.8%; P=.4), there was a difference in inappropriate interventions (8.2%, 7.1%, and 12.0%, respectively; P=.03).
ConclusionsAll-cause and cardiovascular mortality in patients with valvular cardiomyopathy were similar to those in other patients referred for defibrillator implantation. They also had similar rates of appropriate interventions. These data suggest that defibrillator implantation in this patient group confers a similar benefit to that obtained by patients with ischemic or dilated cardiomyopathy.
Keywords
Current evidence indicates that implantable cardioverter-defibrillators (ICDs) reduce mortality in patients with ischemic and nonischemic dilated cardiomyopathy.1–5 However, individuals with left ventricular dysfunction secondary to valve disease are underrepresented in this subgroup, and the outcome data for these patients are less robust.6
The aim of the present study was to describe the outcomes of patients with acute left ventricular dysfunction secondary to valve disease referred for ICD implantation for primary prevention of sudden death compared with those of patients with “ischemic cardiomyopathy” or “dilated cardiomyopathy”.
METHODSThis multicenter retrospective study was performed in 15 Spanish centers with experience in ICD implantation and follow-up of these patients. Consecutive patients with first implantations for primary prevention from 2010 and 2011 were included, as well as consecutive patients from 3 centers with implantations performed after 1 January 2008.
Inclusion CriteriaThe present study included patients referred for defibrillator implantation for primary prevention with or without resynchronization therapy according to current recommendations.7
Exclusion CriteriaThe following patients were excluded: those with channelopathies, arrhythmogenic right ventricular dysplasia, hypertrophic cardiomyopathy, and congenital heart disease.
Definitions of the VariablesCardiovascular mortality included sudden death and death attributed to myocardial infarction or heart failure. Sudden death was defined as death in the first hour after symptom onset or unexpected death in a person who lived alone and was feeling well within the previous 24hours. Death due to refractory heart failure was defined as death in a patient with decompensated heart failure who failed to respond to medical treatment, in the absence of any other cause of death. Death due to myocardial infarction was considered to have occurred when the infarction caused electrical or mechanical complications leading to early death. The remaining deaths were considered noncardiac deaths.
Finally, valvular cardiomyopathy was defined as cardiomyopathy secondary to replacement or repair of the aortic or mitral valve or severe valve disease causing ventricular dysfunction in the absence of other causes explaining the dysfunction before device implantation.8
Baseline Risk StratificationThe MADIT9 and SHOCKED10 scales were used to stratify the baseline risk of the population. The variables included in these scales and their scores are summarized in Table 1.
Variables Included in the 3 Models Used for Predicting Mortality and the Scoring Systems
| Scale | Variables | Categories |
|---|---|---|
| MADIT II | Age > 70 years (1 point) QRS width > 120ms (1 point) AF (1 point) NYHA > II (1 point) BUN > 26 mg/dL (1 point) | Low risk: score of 0 Intermediate risk: score of 1 or 2 High risk: score ≥ 3 |
| SHOCKED | Age ≥ 75 years (62 points) NYHA III (36 points) AF (27 points) COPD (62 points) Chronic kidney disease (100 points) LVEF ≤ 20% (28 points) Diabetes mellitus (41 points) | The abbreviated model equation is represented in the form of nomograms with a maximum of 360 points. The rates of mortality are based on risk quintiles The highest risk quintile is > 202 |
AF, atrial fibrillation; BUN, blood urea nitrogen; COPD, chronic obstructive pulmonary disease; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association.
Gaussian continuous variables in an initial descriptive analysis are expressed as mean and standard deviation and non-Gaussian continuous variables as median (minimum and maximum). To identify differences among the study groups (valvular, ischemic, and dilated cardiomyopathy), the parametric analysis of variance test was used for Gaussian variables and the Kruskal-Wallis test was used for non-Gaussian variables. Categorical variables were compared with a chi-square test. Kaplan-Meier life table and competitive risk analyses were performed. All-cause mortality and cardiovascular mortality were calculated for each type of cardiomyopathy using Cox regression models. In these models, p-spline smoothing was used for continuous variables to determine the linearity of the variables. Various multivariate Cox models were used to identify the best explanatory model and the model with the highest plausibility or Atkinson R2 test and best c-statistic was selected. Cox regression models were used to analyze appropriate and inappropriate ICD interventions (presence or absence). The following variables were included: age, sex, MADIT and SHOCKED scales, peripheral arterial disease, diabetes mellitus, left ventricular ejection fraction, New York Heart Association functional class, chronic obstructive pulmonary disease, dyslipidemia, obstructive sleep apnea syndrome, history of atrial fibrillation, QRS width, creatinine clearance, heart rate at the time of implantation, blood urea nitrogen, bundle branch block at the time of implantation, hypertension, ischemic cardiomyopathy, incomplete revascularization, body mass index, and use of beta-blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, statins, antialdosterone agents, and digoxin. P values were obtained with a 2-tailed test, and P<.05 was considered statistically significant. All statistical analyses were performed using SPSS 19 and Stata 13 (stcompet) software and the free software R (survival package).
For the univariate analysis, the category “ischemic cardiomyopathy” was considered a reference subcategory for the explanatory variable “type of cardiomyopathy”.
RESULTSOf the 1185 patients included in this study, 11 were excluded due to missing follow-up data (final n=1174). Patients with hypertrophic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy, or channelopathies were not included in the database and it is unknown how many were excluded for these reasons. The date of implantation was before 2010 in 281 of the 1174 patients (in 3 specific centers: Hospital Clínico Universitario de Santiago de Compostela, Hospital Universitario Marqués de Valdecilla, and Hospital Clínico Universitario de Valencia); in the other patients, the date of implantation was after 2010 (n=893). There were no relevant differences between the risk profiles of the patients implanted before and after 2010 or among the proportion of cardiomyopathy type in each category (25% ischemic, 18% valvular, and 21% dilated; P=.1).
Baseline CharacteristicsOf the 1174 patients included in the study, 73 had valvular dilated cardiomyopathy (10 patients also had coronary disease with consequent complete revascularization but the dysfunction was attributed to valve disease).
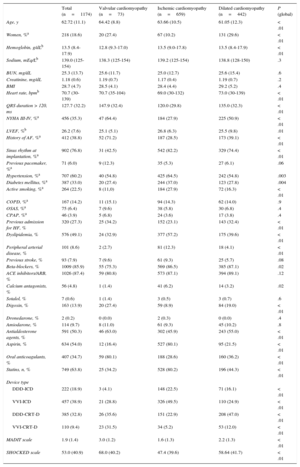
The baseline characteristics of the overall population and cardiomyopathy subtypes are shown in Table 2; notably, patients with valvular cardiomyopathy showed worse functional class, wider QRS, and a history of atrial fibrillation.
Baseline Characteristics of the Overall Population and by Cardiomyopathy Subgroup. In the Last Column, the Differences Among the 3 Groups Were Calculated Using the Analysis of Variance Test
| Total (n=1174) | Valvular cardiomyopathy (n=73) | Ischemic cardiomyopathy (n=659) | Dilated cardiomyopathy (n=442) | P (global) | |
|---|---|---|---|---|---|
| Age, y | 62.72 (11.1) | 64.42 (8.8) | 63.66 (10.5) | 61.05 (12.3) | < .01 |
| Women, %a | 218 (18.6) | 20 (27.4) | 67 (10.2) | 131 (29.6) | < .01 |
| Hemoglobin, g/dLb | 13.5 (8.4-17.9) | 12.8 (9.3-17.0) | 13.5 (9.0-17.8) | 13.5 (8.4-17.9) | < .01 |
| Sodium, mEq/Lb | 139.0 (125-154) | 138.3 (125-154) | 139.2 (125-154) | 138.8 (128-150) | .3 |
| BUN, mg/dL | 25.3 (13.7) | 25.6 (11.7) | 25.0 (12.7) | 25.6 (15.4) | .6 |
| Creatinine, mg/dL | 1.18 (0.6) | 1.19 (0.7) | 1.17 (0.4) | 1.19 (0.7) | .2 |
| BMI | 28.7 (4.7) | 28.5 (4.1) | 28.4 (4.4) | 29.2 (5.2) | .4 |
| Heart rate, bpmb | 70.7 (30-139) | 70.7 (35-104) | 69.0 (30-132) | 73.0 (30-139) | < .01 |
| QRS duration > 120, ms | 127.7 (32.2) | 147.9 (32.4) | 120.0 (29.8) | 135.0 (32.3) | < .01 |
| NYHA III-IV, %a | 456 (35.3) | 47 (64.4) | 184 (27.9) | 225 (50.9) | < .01 |
| LVEF, %b | 26.2 (7.6) | 25.1 (5.1) | 26.8 (6.3) | 25.5 (9.8) | .01 |
| History of AF, %a | 412 (38.8) | 52 (71.2) | 187 (28.5) | 173 (39.1) | < .01 |
| Sinus rhythm at implantation, %a | 902 (76.8) | 31 (42.5) | 542 (82.2) | 329 (74.4) | < .01 |
| Previous pacemaker, %a | 71 (6.0) | 9 (12.3) | 35 (5.3) | 27 (6.1) | .06 |
| Hypertension, %a | 707 (60.2) | 40 (54.8) | 425 (64.5) | 242 (54.8) | .003 |
| Diabetes mellitus, %a | 387 (33.0) | 20 (27.4) | 244 (37.0) | 123 (27.8) | .004 |
| Active smoking, %a | 264 (22.5) | 8 (11,0) | 184 (27.9) | 72 (16.3) | < .01 |
| COPD, %a | 167 (14.2) | 11 (15.1) | 94 (14.3) | 62 (14.0) | .9 |
| OSAS, %a | 75 (6.4) | 7 (9.6) | 38 (5.8) | 30 (6.8) | .4 |
| CPAP, %a | 46 (3.9) | 5 (6.8) | 24 (3.6) | 17 (3.8) | .4 |
| Previous admission for HF, % | 320 (27.3) | 25 (34.2) | 152 (23.1) | 143 (32.4) | < .01 |
| Dyslipidemia, % | 576 (49.1) | 24 (32.9) | 377 (57.2) | 175 (39.6) | < .01 |
| Peripheral arterial disease, % | 101 (8.6) | 2 (2.7) | 81 (12.3) | 18 (4.1) | < .01 |
| Previous stroke, % | 93 (7.9) | 7 (9.6) | 61 (9.3) | 25 (5.7) | .08 |
| Beta-blockers, % | 1009 (85.9) | 55 (75.3) | 569 (86.5) | 385 (87.1) | .02 |
| ACE inhibitors/ARB, % | 1026 (87.4) | 59 (80.8) | 573 (87.1) | 394 (89.1) | .12 |
| Calcium antagonists, % | 56 (4.8) | 1 (1.4) | 41 (6.2) | 14 (3.2) | .02 |
| Sotalol, % | 7 (0.6) | 1 (1.4) | 3 (0.5) | 3 (0.7) | .6 |
| Digoxin, % | 163 (13.9) | 20 (27.4) | 59 (8.9) | 84 (19.0) | < .01 |
| Dronedarone, % | 2 (0.2) | 0 (0.0) | 2 (0.3) | 0 (0.0) | .4 |
| Amiodarone, % | 114 (9.7) | 8 (11.0) | 61 (9.3) | 45 (10.2) | .8 |
| Antialdosterone agents, % | 591 (50.3) | 46 (63.0) | 302 (45.9) | 243 (55.0) | < .01 |
| Aspirin, % | 634 (54.0) | 12 (16.4) | 527 (80.1) | 95 (21.5) | < .01 |
| Oral anticoagulants, % | 407 (34.7) | 59 (80.1) | 188 (28.6) | 160 (36.2) | < .01 |
| Statins, n, % | 749 (63.8) | 25 (34.2) | 528 (80.2) | 196 (44.3) | < .01 |
| Device type | |||||
| DDD-ICD | 222 (18.9) | 3 (4.1) | 148 (22.5) | 71 (16.1) | < .01 |
| VVI-ICD | 457 (38.9) | 21 (28.8) | 326 (49.5) | 110 (24.9) | < .01 |
| DDD-CRT-D | 385 (32.8) | 26 (35.6) | 151 (22.9) | 208 (47.0) | < .01 |
| VVI-CRT-D | 110 (9.4) | 23 (31.5) | 34 (5.2) | 53 (12.0) | < .01 |
| MADIT scale | 1.9 (1.4) | 3.0 (1.2) | 1.6 (1.3) | 2.2 (1.3) | < .01 |
| SHOCKED scale | 53.0 (40.9) | 68.0 (40.2) | 47.4 (39.6) | 58.64 (41.7) | < .01 |
ACE, angiotensin-converting enzyme; AF, atrial fibrillation; ARB, angiotensin receptor blockers; BMI, body mass index; BUN, blood urea nitrogen; COPD, chronic obstructive pulmonary disease; CPAP, continuous positive airway pressure; CRT-D, cardiac resynchronization therapy-defibrillator; HF, heart failure; ICD, implantable cardioverter-defibrillator; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; OSAS, obstructive sleep apnea syndrome.
Significant drug treatment-related differences were found according to cardiomyopathy type. Overall, 85.9% of the patients received beta-blockers (86.5% of those with ischemic cardiomyopathy, 87.1% with dilated cardiomyopathy, and 75.3% with valvular cardiomyopathy; P=.02) and 4.8% were treated with calcium antagonists (6.2% of those with ischemic cardiomyopathy, 3.2% with dilated cardiomyopathy, and 1.4% with valvular cardiomyopathy; P=.02). Digoxin was taken by 13.9% (27.4% of those with valvular cardiomyopathy, 19.0% with dilated cardiomyopathy, and 8.9% with ischemic cardiomyopathy; P<.01). Significant differences were seen in antialdosterone use among the 3 groups (63,0% of those with valvular cardiomyopathy, 55.0% with dilated cardiomyopathy, and 45.9% with ischemic cardiomyopathy; P<.01). Acetylsalicylic acid was taken by 54.0% of patients (80.1% of those with ischemic cardiomyopathy, 16.4% with valvular cardiomyopathy, and 21.5% with dilated cardiomyopathy; P<.01). Oral anticoagulants were taken by 34.7% and their use was significantly higher in those with valvular cardiomyopathy (80.1% vs 28.6% with ischemic cardiomyopathy and 36.2% with dilated cardiomyopathy; P<.01 (Table 2).
Patients with valvular cardiomyopathy had a worse risk profile according to the MADIT (mean and standard deviation scores of the valves) and SHOCKED (idem) scales than the ischemic cardiomyopathy or dilated cardiomyopathy groups.
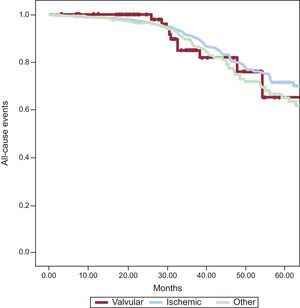
Overall and Cardiovascular Mortality According to Cardiomyopathy SubgroupDuring a mean follow-up of 38.1±21.3 months, 197 patients (16.7%) died: 14 (19.2%) of those with valvular cardiomyopathy, 104 (15.8%) with ischemic cardiomyopathy, and 79 (17.9%) with dilated cardiomyopathy (P=.2) (Figure).
The cause of death was cardiovascular in 136 of the 197 deaths (11.6%): 9 patients (12.3%) with valvular cardiomyopathy, 69 (10.5%) with ischemic cardiomyopathy, and 58 (13.1%) with dilated cardiomyopathy (P=.1).
The crude effect of the analyzed variables on the event “all-cause mortality” is shown in Table 3.
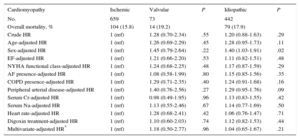
Crude and Adjusted Effect of the Type of Idiopathic Valvular and Ischemic Cardiomyopathy on Overall Mortality in Patients Receiving an Implantable Cardioverter-Defibrillator for Primary Prevention. The Overall Mortality of Each Cardiomyopathy Was Compared with the Overall Mortality in Patients with Ischemic Cardiomyopathy (Reference)
| Cardiomyopathy | Ischemic | Valvular | P | Idiopathic | P |
|---|---|---|---|---|---|
| No. | 659 | 73 | 442 | ||
| Overall mortality, % | 104 (15.8) | 14 (19.2) | 79 (17.9) | ||
| Crude HR | 1 (ref) | 1.28 (0.70-2.34) | .55 | 1.20 (0.88-1.63) | .29 |
| Age-adjusted HR | 1 (ref) | 1.26 (0.69-2.29) | .45 | 1.28 (0.95-1.73) | .11 |
| Sex-adjusted HR | 1 (ref) | 1.45 (0.79-2.64) | .22 | 1.40 (1.03-1.91) | .02 |
| EF-adjusted HR | 1 (ref) | 1.21 (0.66-2.20) | .53 | 1.11 (0.82-1.51) | .48 |
| NYHA functional class-adjusted HR | 1 (ref) | 1.24 (0.68-2.25) | .48 | 1.17 (0.87-1.59) | .29 |
| AF presence-adjusted HR | 1 (ref) | 1.08 (0.58-1.99) | .80 | 1.15 (0.85-1.56) | .35 |
| COPD presence-adjusted HR | 1 (ref) | 1.29 (0.71-2.35) | .40 | 1.24 (0.91-1.68) | .16 |
| Peripheral arterial disease-adjusted HR | 1 (ref) | 1.40 (0.76-2.56) | .27 | 1.29 (0.95-1.76) | .09 |
| Serum Cr-adjusted HR | 1 (ref) | 0.98 (0.49-1.95) | .96 | 1.13 (0.83-1.55) | .42 |
| Serum Na-adjusted HR | 1 (ref) | 1.13 (0.55-2.46) | .67 | 1.14 (0.77-1.69) | .50 |
| Heart rate-adjusted HR | 1 (ref) | 1.28 (0.68-2.41) | .42 | 1.06 (0.76-1.47) | .71 |
| Digoxin treatment-adjusted HR | 1 (ref) | 1.10 (0.60-2.03) | .74 | 1.12 (0.82-1.53) | .44 |
| Multivariate-adjusted HR* | 1 (ref) | 1.18 (0.50-2.77) | .96 | 1.04 (0.65-1.67) | .21 |
95%CI, 95% confidence interval; AF, atrial fibrillation; COPD, chronic obstructive pulmonary disease; Cr, creatinine; EF, ejection fraction; HR, hazard ratio; Na, sodium; NYHA, New York Heart Association functional class; ref, reference.
* Cox regression analysis adjusted by age, sex, ejection fraction, chronic obstructive pulmonary disease creatinine, and digoxin treatment, all at the time of implant.
In the adjusted analysis, the independent predictors of all-cause mortality were left ventricular ejection fraction on admission (hazard ratio [HR]=0.96; 95% confidence interval [95%CI], 0.93-0.97), age (HR=1.03; 95%CI, 1.01-1.06), serum creatinine (HR=1.66; 95%CI, 1.36-2.02), male sex (HR=2.5; 95%CI, 1.27-4.95), chronic obstructive pulmonary disease (HR=1.73; 95%CI, 1.09-2.75), digoxin therapy (HR=1.61; 95%CI, 1.02-2.53), with a c-statistic for this model of 0.71. No relevant changes were seen with a specific analysis of cardiovascular mortality, considering the left ventricular ejection fraction on admission (HR=0.96; 95%CI, 0.94-0.98), age (HR=1.02; 95%CI, 1.01-1.04), serum creatinine (HR=1.58; 95%CI, 1.30-1.91), male sex (HR=2.35; 95%CI, 1.26-4.38), obstructive pulmonary disease (HR=1.64; 95%CI, 1.08-2.48), and digoxin therapy (HR=1.84; 95%CI, 1.23-2.76). The type of cardiomyopathy was not significant in the univariate analysis or in any of the multivariate analyses performed, whether for all-cause mortality or cardiovascular mortality. Regression analysis ruled out the presence of competitive risks.
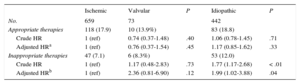
Appropriate and Inappropriate Therapies According to SubgroupDuring the study period, 211 patients (18%) received an appropriate device intervention (antitachycardia pacing, shock, or both): 10 patients (13.7%) with valvular cardiomyopathy, 118 (17.9%) with ischemic cardiomyopathy, and 83 (18.7%) with dilated cardiomyopathy (P=.4). In the crude analysis, the only variable associated with a lower risk of appropriate therapy was beta-blockers (HR=0.63; 95CI%, 0.42-0.93; P=.02). However, for the type of cardiomyopathy variable, after adjustment for age, sex, and beta-blocker therapy, there was no significantly increased risk of appropriate therapy in patients with valvular or nonischemic cardiomyopathy (HR=0.76; 95CI%, 0.37-1.54; P=.4) vs those with ischemic cardiomyopathy (HR=1.17; 95CI%, 0.85-1.62; P=.3) (Table 4).
Association Between Cardiomyopathy Type and the Incidence of Appropriate and Inappropriate Therapies of Implantable Cardioverter-defibrillators
| Ischemic | Valvular | P | Idiopathic | P | |
|---|---|---|---|---|---|
| No. | 659 | 73 | 442 | ||
| Appropriate therapies | 118 (17.9) | 10 (13.9%) | 83 (18.8) | ||
| Crude HR | 1 (ref) | 0.74 (0.37-1.48) | .40 | 1.06 (0.78-1.45) | .71 |
| Adjusted HRa | 1 (ref) | 0.76 (0.37-1.54) | .45 | 1.17 (0.85-1.62) | .33 |
| Inappropriate therapies | 47 (7.1) | 6 (8.3%) | 53 (12.0) | ||
| Crude HR | 1 (ref) | 1.17 (0.48-2.83) | .73 | 1.77 (1.17-2.68) | < .01 |
| Adjusted HRb | 1 (ref) | 2.36 (0.81-6.90) | .12 | 1.99 (1.02-3.88) | .04 |
HR, hazard ratio.
Inappropriate ICD interventions were seen in 106 patients (9%): 6 (8.2%) with valvular cardiomyopathy, 47 (7.1%) with ischemic cardiomyopathy, and 53 (12.0%) with dilated cardiomyopathy (P=.4). In the multivariate Cox analysis, the variables associated with inappropriate therapies were age < 65 years (HR=2.58; 95%CI, 1.28-5.22; P<.01), history of atrial fibrillation (HR=2.25; 95%CI, 1.23-4.11; P<.01), and cardiac resynchronization therapy (HR=0.38; 95%CI, 0.20-0.74; P<.01). No significantly higher risk of inappropriate therapy was found in patients with valvular cardiomyopathy (HR=2.36; 95%CI, 0.81-6.90; P=.12), but a significantly higher risk was seen in those with nonischemic cardiomyopathy (HR=1.99; 95%CI, 1.02-3.88; P=.01) vs the reference group of patients with ischemic cardiomyopathy after adjustment for age, sex, left ventricular ejection fraction, heart rate, hypertension, chronic obstructive pulmonary disease, and peripheral arterial disease (Table 4).
DISCUSSIONIn the present cohort, no notable differences were seen in the rates of all-cause and cardiovascular mortality in patients with left ventricular dysfunction of different causes who received an ICD implantation for primary prevention. There were also no significant differences in the rate of appropriate device interventions, even though these patients showed a more adverse clinical risk profile according to the event prediction scores (MADIT and SHOCKED scales). Thus, patients with valvular cardiomyopathy appear to obtain the same benefit as the rest of the population referred for ICD implantation for primary prevention.
The main objective of this study was to describe the outcomes of this particular population, given that current evidence supporting the use of an ICD for preventing arrhythmic death in patients with left ventricular dysfunction secondary to valve disease is insufficiently robust.1–5 In fact, this patient type is generally underrepresented in the large randomized studies,6 and the present work represents one of the largest series available to date. However, evidence indicates that these patients show a substantial rate of arrhythmic events, particularly after valve replacement,11–14 reaching 25% in some series,12–14 which can be explained by various mechanisms. It is first important to note the presence of left ventricular hypertrophy, dilatation, and left ventricular dysfunction in this type of pathology.14–16 Moreover, fibrosis after surgery has been suggested to provide a favorable substrate for the creation of reentry phenomena and consequent ventricular arrhythmias.13 In fact, in the series reported by Rosenheck et al,17 antitachycardia pacing was effective in 99% of the ventricular tachycardia episodes, suggesting that reentry would be the main mechanism of ventricular arrhythmia in this population. These characteristics might explain the significant rate of cardiovascular events, which was similar to that of patients with ischemic cardiomyopathy, for example, independently of the correction of the underlying valve disease.
Valles et al15 published a series of 31 patients with valvular cardiomyopathy and ICD implantation for primary and secondary prevention. In this series, there were no differences (in survival and appropriate discharges) compared with patients with ischemic cardiomyopathy and a control group, although there was a tendency for worse outcomes in patients with ischemic cardiomyopathy. Rosenheck et al17 described 31 patients with valvular cardiomyopathy and primary and secondary prevention (of a total of 438 individuals). As is inherent to this group, and as in our population, the baseline profile of the patients was different from those with ischemic cardiomyopathy. Although there was a slight tendency for an overall higher cumulative survival than in those patients with ischemic cardiomyopathy and dilated cardiomyopathy, there were no significant differences.
These data, together with the results of our series, seem to indicate that this subgroup of patients can benefit from primary prevention with an ICD, similar to those with cardiomyopathy of other causes. Such conclusions must be analyzed from the perspective that the patients have different clinical characteristics than those with ischemic cardiomyopathy or dilated cardiomyopathy, as reflected in the higher score in the clinical scales for predicting mortality events (MADIT and SHOCKED). In contrast, these patients show less diabetes, smoking, peripheral arterial disease, and dyslipidemia and, as expected, particularities specific to this group regarding medical treatment (greater use of digoxin and oral anticoagulants), as well significantly wider QRS, which would at least partly explain the significantly larger percentage of implantations of cardiac resynchronization systems.
Finally, another important conclusion from this cohort is that the group of patients with valve diseases did not show a higher rate of inappropriate therapies than patients with ischemic cardiomyopathy (and even showed a lower rate than those with dilated cardiomyopathy). Although the high incidence of atrial fibrillation in patients with valve diseases is presumed to be a risk factor, the current evidence (from our cohort and that of Valles et al15) fails to support this claim.
This fact, together with the previously mentioned results, supports the risk/benefit relationship of ICD implantation for primary prevention in patients with valvular cardiomyopathy, as in patients with ischemic cardiomyopathy or dilated cardiomyopathy. Nonetheless, these findings should be confirmed in a series with a larger number of patients and a more homogenous baseline profile.
LimitationsThis study should be interpreted with the limitations inherent to its retrospective and nonrandomized design. The conclusions should take into account the particularly high percentage of patients in the valvular cardiomyopathy group who were also implanted with a left ventricular electrode for ventricular resynchronization. Therefore, the survival and device discharge rates were probably strongly influenced by resynchronization therapy. However, the proportion of patients with added resynchronization therapy is similar to that in the studies that helped to inform the current guidelines.
Finally, one important limitation is the lack of information on the type of valve disease (aortic, mitral), surgical intervention (valve replacement vs repair), and associated disease (eg, pulmonary hypertension, right ventricular dysfunction), which would surely affect survival and device interventions. Thus, the present work is an initial approach to the study of these patients. These limitations should be taken into account when interpreting its conclusions and by future studies aimed at optimizing the selection criteria of patients with valve diseases referred for an ICD implant.
CONCLUSIONSIn patients with valve-related left ventricular dysfunction, implantation of an ICD seems to confer a benefit similar to that obtained by patients with ischemic cardiomyopathy or dilated cardiomyopathy. The rates of all-cause and cardiovascular mortality were similar to those of the other patients referred for an ICD, without significant differences. The patients also showed similar rates of appropriate device interventions (antitachycardia pacing, shock, or both). However, there were baseline differences in the risk profiles and medical treatment of this population vs patients with ischemic cardiomyopathy or dilated cardiomyopathy. Thus, these conclusions should be carefully interpreted and confirmed in future studies focusing specifically on this heretofore underrepresented population.
CONFLICTS OF INTERESTNone declared.
The authors would like to express their admiration and thanks to Dr. Sáenz de Buruaga for teaching us how to treat patients with valve diseases and for carefully reviewing this manuscript.
Cristina González-Cambeiro, Luis Martínez-Sande, and José Ramón González-Juanatey (Hospital Universitario de Santiago de Compostela, Santiago de Compostela); Óscar Salvador-Montañés (Hospital Universitario La Paz, Madrid); José Moreno-Arribas (Hospital Universitario de San Juan, Sant Joan d’Alacant, Alicante); Agustín Fernández-Cisnal and Alonso Pedrote-Martínez (Hospital Universitario Virgen del Rocío, Sevilla); Juan Benezet-Mazuecos (Hospital Fundación Jiménez Díaz, Madrid); Hugo Arguedas-Jiménez (Clínica Universidad de Navarra, Pamplona, Navarra); Jesús Jiménez-López (Hospital Virgen de la Salud, Toledo); Juan José Olalla Antolín (Hospital Universitario Marqués de Valdecilla, Santander, Cantabria); Ricardo Ruiz-Granell (Hospital Clínico Universitario de Valencia); Jose Ormaetxe (Hospital de Basurto, Bilbao, Vizcaya); Enrique García (Hospital Universitario de Vigo, Vigo, Pontevedra); Jose Olagüe de Ros (Hospital Universitario y Politécnico La Fe, Valencia), and Luis Tercedor-Sánchez (Hospital Virgen de las Nieves, Granada).