Posttransplant outcomes among recipients with a diagnosis of hypertrophic cardiomyopathy (HCM) or restrictive cardiomyopathy (RCM) remain controversial.
MethodsRetrospective analysis of a nationwide registry of first-time recipients undergoing isolated heart transplant between 1984 and 2021. One-year and 5-year mortality in recipients with HCM and RCM were compared with those with dilated cardiomyopathy (DCM).
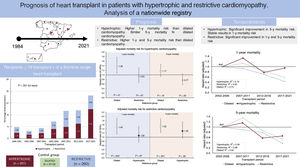
ResultsWe included 3703 patients (3112 DCM; 331 HCM; 260 RCM) with a median follow-up of 5.0 [3.1-5.0] years. Compared with DCM, the adjusted 1-year mortality risk was: HCM: HR, 1.38; 95%CI, 1.07-1.78; P=.01, RCM: HR, 1.48; 95%CI, 1.14-1.93; P=.003. The adjusted 5-year mortality risk was: HCM: HR, 1.17; 95%CI, 0.93-1.47; P=.18; RCM: HR, 1.52; 95%CI, 1.22-1.89; P<.001. Over the last 20 years, the RCM group showed significant improvement in 1-year survival (adjusted R2=0.95) and 5-year survival (R2=0.88); the HCM group showed enhanced the 5-year survival (R2=0.59), but the 1-year survival remained stable (R2=0.16).
ConclusionsBoth RCM and HCM were linked to a less favorable early posttransplant prognosis compared with DCM. However, at the 5-year mark, this unfavorable difference was evident only for RCM. Notably, a substantial temporal enhancement in both early and late mortality was observed for RCM, while for HCM, this improvement was mainly evident in late mortality.
Keywords
Dilated cardiomyopathy remains the most common indication for heart transplant (HT), comprising more than 80% of procedures. The primary etiology of cardiomyopathy leading to transplantation can affect pretransplant management1–7 and posttransplant outcomes,8–12 influencing decisions about waiting lists and donor allocation policies. Indeed, the recently modified adult heart allocation system policies of the OPTN/UNOS13 have assigned some degree of prioritization in the waiting list for less frequent indications such as hypertrophic cardiomyopathy (HCM) and restrictive cardiomyopathy (RCM) considering the technical difficulties in providing appropriate mechanical circulatory support and the limited usefulness and safety concerns of inotropic support in the end-stage phase of these diseases. Cardiac recipients with amyloid heart disease have a poor posttransplant prognosis,9,14 particularly if the primary disease is left untreated.15 However, outcomes are likely to have improved in the contemporary era due to better patient selection and pretransplant care, as well as improved treatments targeting the underlying disease.16 Available data show that amyloid RCM have worse posttransplant results than other etiologies.17 Some single-center studies have reported favorable survival after HT in HCM.18–20 These findings seem to be confirmed by several, but not all, large registry-based studies.6,7,21,22
This study aimed to analyze HT outcomes in adults with HCM or RCM over the past 2 decades. We also explored differences within each etiology: comparing amyloidosis vs nonamyloidosis in RCM and nonreduced vs reduced left ventricular ejection fraction (LVEF) in HCM.
METHODSData sourceWe conducted a retrospective analysis of the Spanish Registry of Heart Transplantation (SRHT), a prospective registry that has enrolled all patients receiving a transplant in Spain since the initiation of this activity in May, 1984. Data on recipient demographics, baseline clinical characteristics, donor characteristics, transplant procedure and immunosuppression, as well as outcomes, were collected from the registry database. Vital status was ascertained through December 31, 2021.
The SRHT is a comprehensive registry that encompasses all the transplant centers across Spain and includes all the patients who have undergone HT since 1984. Centers are linked to a public, nonprofit national health system. These centers have established a stringent protocol for patient monitoring and follow-up, benefitting from patients’ notable commitment to the health system. Data are entered by each center using a predefined form hosted within a specific application accessible via the internet. This procedure is supervised by a contract research organization and is under the oversight of the medical direction of the registry. Details about donors are supplied by the National Transplant Organization. Annually, in January, each center logs the previous year's transplants, and clinical data are regularly updated. Vital status checks are mandatory for new and historical cases.
All patients granted written informed consent upon their inclusion on the transplant waiting list, allowing the use of their deidentified data for research purposes. Additionally, the SRHT operations have received approval from the ethic committee of Hospital Universitario La Fe de Valencia (Spain).
Study populationWe included patients aged 18 and older who underwent their first isolated HT between May 1984 and December 2021. The study focused on patients diagnosed with HCM or RCM. In the RCM group, we included patients with amyloidosis, as well as those with idiopathic/undefined, radiation/chemotherapy, endocardial fibrosis, and sarcoidosis etiologies. The comparison group comprised patients with a pretransplant diagnosis of dilated cardiomyopathy (DCM), which encompassed individuals with idiopathic/undefined, chemotherapy-induced, postpartum, familial, and toxic etiologies.
OutcomesThe primary outcome was all-cause mortality at 1 year, with 5-year all-cause mortality as the secondary outcome. Additionally, we analyzed specific causes of death within the first year, the incidence of primary graft failure, duration of posttransplant mechanical ventilation, and intensive care unit length of stay.
Missing dataThe quantity and percentage of these missing values, categorized by the study variables, can be found in table 1 of the supplementary data. In aggregate, 4.3% of the complete dataset had missing information. Variables exceeding 20% of missing data were excluded. For the remaining variables, a multiple imputation approach was used utilizing chained equations through an iterative Markov Chain Monte Carlo algorithm. Imputation was conducted using linear regression for quantitative variables, logistic regression for dichotomous variables, and multinomial logistic regression for categorical variables. In this process, all study variables and the outcome variable were included as independent variables. The regression models were executed on each of the 15 imputed datasets, and the outcomes were averaged using Rubin's rules.23
Statistical analysisQuantitative variables are summarized as median [interquartile range]. Categorical variables are summarized as frequency (percentage). Differences among groups were analyzed using the Kruskal Wallis rank test or the Wilcoxon rank-sum test for continuous variables, and with the chi-square test for categorical variables. Bonferroni's correction was used for post hoc pairwise comparisons.
Survival was analyzed using the Kaplan-Meier method, and survival curves were compared using the log-rank test. Hazard ratios (HR) with 95% confidence intervals (95%CI) were estimated using Cox regression. Proportional hazard assumption was assessed graphically using log-log plots. Multivariable analyses included, apart from primary etiology, all variables that reached a P-value<.1 in the univariable analysis.24 A sensitivity analysis was conducted wherein the variable of “high priority transplantation” was excluded from the multivariable regression models.
To evaluate temporal trends in mortality risk, the past 20 years (2002-2021) were categorized into 4-year intervals, with the 2002 to 2006 period serving as the reference group. A linear regression was conducted between the HR and the transplant era, incorporating a weighted approach based on sample size and including an intercept.25 The adjusted R-squared value was employed to quantify the strength of the regression. The significance level of 2-tailed tests was set at α <.05. The analysis was performed using Stata 16.0.
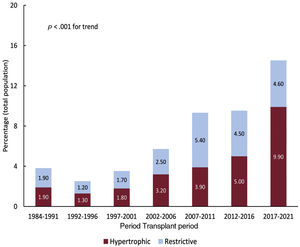
RESULTSPopulation characteristicsBetween May 1984 and December 2021, 8351 patients met the inclusion criteria. After we excluded etiologies not relevant to the present analysis, the study population comprised 3703 patients (median age, 53 [17] years; 991 females [26.8%]). The study groups were composed of 331 recipients with HCM and 260 recipients with RCM (comprising 104 patients with idiopathic RCM, 90 with amyloidosis, 19 with endocardial fibrosis, 5 with sarcoidosis, 10 with radiation/chemotherapy-related cardiomyopathy, and 32 with other causes). The reference group consisted of 3112 recipients with DCM. The percentage changes in the study groups relative to the entire transplant population are illustrated in figure 1. Approximately 57.4% of HCM transplants and 43.8% of RCM transplants occurred between 2012 and 2021. Indeed, during the last 5 years, HCM and RCM have collectively accounted for nearly one-seventh of all cases of transplant.
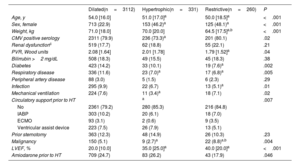
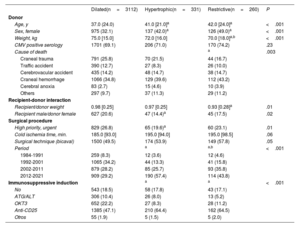
The characteristics of the study groups are summarized in table 1 and table 2. The HCM and RCM groups displayed significant differences from the DCM group. These differences encompassed being younger, having a higher percentage of females, and showing a higher LVEF. For the remaining recipient characteristics, the differences compared with the DCM group varied depending on the specific study group. The HCM group had lower rates of cytomegalovirus (CMV) seropositivity, respiratory disease, pretransplant mechanical ventilation, circulatory support with extracorporeal membrane oxygenation, and pretransplant malignancies than the DCM group. In contrast, the RCM group exhibited lower pulmonary vascular resistance and body weight, lower percentages of diabetes, respiratory disease, and infection, and a higher pretransplant malignancy rate. The HCM and RCM groups differed only in terms of body weight (lower for RCM) and the rate of pretransplant malignancy (higher for RCM). Overall, the use of ventricular assist devices before HT was comparable across all groups. Nevertheless, this use showed an upward trend in the last decade, being more frequent in the DCM (21.9%) than in the HCM (13.4%) and RCM (9.7%) groups (P=.001).
Recipient characteristics according to the etiology of the underlying heart disease (1984-2021)
| Dilated(n=3112) | Hypertrophic(n=331) | Restrictive(n=260) | P | |
|---|---|---|---|---|
| Age, y | 54.0 [16.0] | 51.0 [17.0]a | 50.0 [18.5]a | <.001 |
| Sex, female | 713 (22.9) | 153 (46.2)a | 125 (48.1)a | <.001 |
| Weight, kg | 71.0 [18.0] | 70.0 [20.0] | 64.5 [17.5]a,b | <.001 |
| CMV positive serology | 2311 (79.9) | 236 (73.3)a | 201 (80.1) | .02 |
| Renal dysfunctionc | 519 (17.7) | 62 (18.8) | 55 (22.1) | .21 |
| PVR, Wood units | 2.08 [1.64] | 2.01 [1.78] | 1.79 [1.52]a | .04 |
| Bilirrubin >2 mg/dL | 508 (18.3) | 49 (15.5) | 45 (18.3) | .38 |
| Diabetes | 423 (14.2) | 33 (10.1) | 19 (7.6)a | .002 |
| Respiratory disease | 336 (11.6) | 23 (7.0)a | 17 (6.8)a | .005 |
| Peripheral artery disease | 88 (3.0) | 5 (1.5) | 6 (2.3) | .29 |
| Infection | 295 (9.9) | 22 (6.7) | 13 (5.1)a | .01 |
| Mechanical ventilation | 224 (7.6) | 11 (3.4)a | 18 (7.1) | .02 |
| Circulatory support prior to HT | a | .007 | ||
| No | 2361 (79.2) | 280 (85.3) | 216 (84.8) | |
| IABP | 303 (10.2) | 20 (6.1) | 18 (7.0) | |
| ECMO | 93 (3.1) | 2 (0.6) | 9 (3.5) | |
| Ventricular assist device | 223 (7.5) | 26 (7.9) | 13 (5.1) | |
| Prior sternotomy | 363 (12.3) | 48 (14.9) | 26 (10.3) | .23 |
| Malignancy | 150 (5.1) | 9 (2.7)a | 22 (8.8)a,b | .004 |
| LVEF, % | 20.0 [10.0] | 35.0 [25.0]a | 40.0 [20.0]a | <.001 |
| Amiodarone prior to HT | 709 (24.7) | 83 (26.2) | 43 (17.9) | .046 |
CMV, cytomegalovirus; ECMO, extracorporeal membrane oxygenation; HT, heart transplant; IABP, intra-aortic balloon pump; LVEF, left ventricular ejection fraction; PVR, pulmonary vascular resistance.
Data are expressed as No. (%) or median [interquartile range].
Donor, surgical procedure, and immunosuppressive induction characteristics (1984-2021)
| Dilated(n=3112) | Hypertrophic(n=331) | Restrictive(n=260) | P | |
|---|---|---|---|---|
| Donor | ||||
| Age, y | 37.0 (24.0) | 41.0 [21.0]a | 42.0 [24.0]a | <.001 |
| Sex, female | 975 (32.1) | 137 (42.0)a | 126 (49.0)a | <.001 |
| Weight, kg | 75.0 [15.0] | 72.0 [16.0] | 70.0 [18.0]a,b | <.001 |
| CMV positive serology | 1701 (69.1) | 206 (71.0) | 170 (74.2) | .23 |
| Cause of death | a | .003 | ||
| Craneal trauma | 791 (25.8) | 70 (21.5) | 44 (16.7) | |
| Traffic accident | 390 (12.7) | 27 (8.3) | 26 (10.0) | |
| Cerebrovacular accident | 435 (14.2) | 48 (14.7) | 38 (14.7) | |
| Craneal hemorrhage | 1066 (34.8) | 129 (39.6) | 112 (43.2) | |
| Cerebral anoxia | 83 (2.7) | 15 (4.6) | 10 (3.9) | |
| Others | 297 (9.7) | 37 (11.3) | 29 (11.2) | |
| Recipient-donor interaction | ||||
| Recipient/donor weight | 0.98 [0.25] | 0.97 [0.25] | 0.93 [0.28]a | .01 |
| Recipient male/donor female | 627 (20.6) | 47 (14.4)a | 45 (17.5) | .02 |
| Surgical procedure | ||||
| High priority, urgent | 829 (26.8) | 65 (19.6)a | 60 (23.1) | .01 |
| Cold ischemia time, min. | 185.0 [93.0] | 195.0 [94.0] | 195.0 [98.5] | .06 |
| Surgical technique (bicaval) | 1500 (49.5) | 174 (53.9) | 149 (57.8) | .05 |
| Period | a | a,b | <.001 | |
| 1984-1991 | 259 (8.3) | 12 (3.6) | 12 (4.6) | |
| 1992-2001 | 1065 (34.2) | 44 (13.3) | 41 (15.8) | |
| 2002-2011 | 879 (28.2) | 85 (25.7) | 93 (35.8) | |
| 2012-2021 | 909 (29.2) | 190 (57.4) | 114 (43.8) | |
| Immunosuppressive induction | a | a | <.001 | |
| No | 543 (18.5) | 58 (17.8) | 43 (17.1) | |
| ATG/ALT | 306 (10.4) | 26 (8.0) | 13 (5.2) | |
| OKT3 | 652 (22.2) | 27 (8.3) | 28 (11.2) | |
| Anti-CD25 | 1385 (47.1) | 210 (64.4) | 162 (64.5) | |
| Otros | 55 (1.9) | 5 (1.5) | 5 (2.0) |
ATG/ALT, antithymocyte globuline/antilymphocytic therapy; anti-CD25, basiliximab or daclizumab.
Data are expressed as No. (%) or median [interquartile range].
Furthermore, the HCM and RCM groups received transplants from donors who were relatively older, with a higher proportion of female donors. Even so, the HCM group exhibited a significant trend toward a lower proportion of transplants performed with an unfavorable recipient/donor sex match. The cause of donor death was less frequently traumatic in the study groups, with statistical significance observed only in the RCM group compared with the DCM group. The HCM group less frequently received an urgent HT, and there was a nonsignificant trend toward longer ischemia times and a greater use of the bicaval technique in the study groups compared with the DCM group. The HCM and RCM groups showed a higher incidence of induction using anti-CD25 antibodies, whereas induction with OKT3 was less common in comparison with the DCM group.
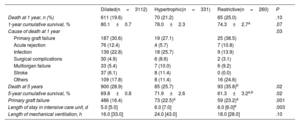
MortalityThe absolute and relative frequencies of all-cause death are summarized in table 3. In total, 746 deaths were observed in the first year, and 1078 deaths occurred by the fifth year of follow-up. Overall, the cumulative survival rate was 79.5%, 69.4%, 58.3% at 1, 5, and 10 years, respectively, with a median survival time of 12.8 years [2.8-22.1].
Main clinical outcomes according to underlying heart disease leading to transplantation
| Dilated(n=3112) | Hypertrophic(n=331) | Restrictive(n=260) | P | |
|---|---|---|---|---|
| Death at 1 year, n (%) | 611 (19.6) | 70 (21.2) | 65 (25.0) | .10 |
| 1-year cumulative survival, % | 80.1±0.7 | 78.0±2.3 | 74.3±2.7a | .07 |
| Cause of death at 1 year | .03 | |||
| Primary graft failure | 187 (30.6) | 19 (27.1) | 25 (38.5) | |
| Acute rejection | 76 (12.4) | 4 (5.7) | 7 (10.8) | |
| Infection | 139 (22.8) | 18 (25.7) | 9 (13.9) | |
| Surgical complications | 30 (4.9) | 6 (8.6) | 2 (3.1) | |
| Multiorgan failure | 33 (5.4) | 7 (10.0) | 6 (9.2) | |
| Stroke | 37 (6.1) | 8 (11.4) | 0 (0.0) | |
| Others | 109 (17.8) | 8 (11.4) | 16 (24.6) | |
| Death at 5 years | 900 (28.9) | 85 (25.7) | 93 (35.8)b | .02 |
| 5-year cumulative survival, % | 69.8±0.8 | 71.9±2.6 | 61.3±3.2a,b | .02 |
| Primary graft failure | 486 (16.4) | 73 (22.5)a | 59 (23.2)a | .001 |
| Length of stay in intensive care unit, d | 5.0 [5.0] | 6.0 [7.0] | 6.0 [6.0]a | .003 |
| Length of mechanical ventilation, h | 16.0 [33.0] | 24.0 [43.0] | 18.0 [28.0] | .10 |
Data are expressed as No. (%), mean±standard deviation, or median [interquartile range].
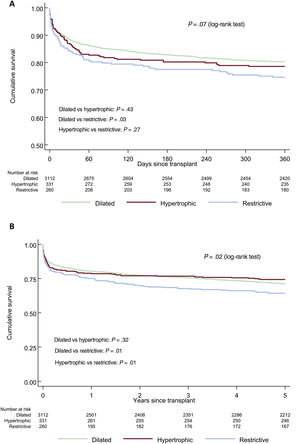
In the unadjusted analysis, 1-year survival was significantly poorer only for RCM compared with DCM (figure 2A). At 5 years, survival was significantly lower in RCM than in either DCM or HCM (figure 2B).
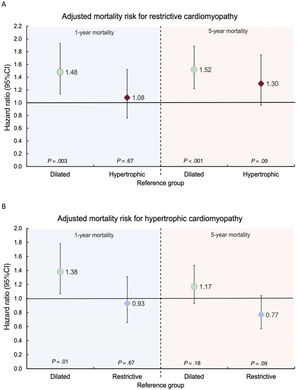
Univariable and multivariable Cox regression analyses for 1-year and 5-year mortality are summarized in tables 2 and 3 of the supplementary data and multivariable adjusted comparisons between groups in table 4 of the supplementary data. The RCM group exhibited a significant 48% higher risk of 1-year mortality than the DCM group (figure 3A). Similarly, the HCM group also demonstrated a significant 38% higher risk of 1-year mortality compared with the DCM group (figure 3B). There were no differences in 1-year mortality between the RCM and HCM groups.
Comparison of adjusted 1-year and 5-year mortality risks between restrictive (A) and hypertrophic (B) cardiomyopathy and dilated cardiomyopathy. One-year mortality risk adjusted for recipient age, renal dysfunction, pulmonary vascular resistance, bilirubin >2mg/dL, infection, mechanical ventilation, pretransplant circulatory support, malignancy, donor sex, urgency, cold ischemia time, transplant era and immunosuppressive induction. Five-year mortality risk adjusted for age, renal dysfunction, pulmonary vascular resistance, bilirubin >2mg/dL, infection, mechanical ventilation, pretransplant circulatory support, prior sternotomy, malignancy, urgency, surgical technique, transplant era and immunosuppressive induction.
Regarding 5-year mortality, the RCM group still showed a significant 52% excess mortality risk compared with the DCM group (figure 3A), whereas the 17% excess mortality risk observed for the HCM group compared with the DCM group was not statistically significant (figure 3B). A nonsignificant trend to higher 5-year mortality risk was observed for the RCM group compared with the HCM group (figure 3A).
These results were not significantly impacted by the level of urgency with which the HT was performed. Thus, excluding the variable “high priority transplantation” yielded unaltered results consistent with those obtained from the original models (table 5 of the supplementary data). In contrast, the use of ventricular assist devices before HT could have influenced the early prognosis of HCM, as in this small subgroup of patients, the HR increased to 1.61 (95%CI, 0.88-3.33) compared with DCM, without statistical significance due to the limited sample size. In the case of RCM, the increase in the HR to 1.61 (95%CI, 0.61-4.19) appeared to be less pronounced due to the already poor overall prognosis in this group.
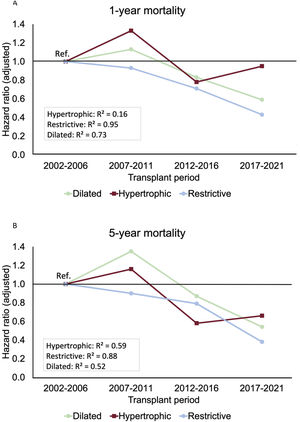
Temporal trendsFigure 4 illustrates the temporal changes in mortality risk in our study groups. There was a significant decrease in 1-year mortality for DCM and RCM, while the HCM group showed little change, maintaining a mortality risk similar to that of the 2002 to 2006 period in the 2017 to 2021 period (figure 4A). Regarding 5-year mortality, all groups showed consistent improvements over time, with noteworthy progress for the RCM and HCM groups (figure 4B).
Cause of death and posttransplant eventsPrimary graft failure occurred in around 23% of the study groups, which was notably higher than in DCM cases. Prolonged mechanical ventilation and an extended intensive care unit stay indicated a more intricate immediate postoperative recovery in HCM and RCM.
While the overall comparison of causes of death was statistically significant, no specific pairwise comparisons were significant (table 3). Notably, the HCM group had more deaths from surgical complications, multiorgan failure, and strokes, but fewer deaths from acute rejection compared with the DCM group. In contrast, the RCM group had more deaths from primary graft failure and multiorgan failure, but fewer deaths from infection and strokes than the DCM group.
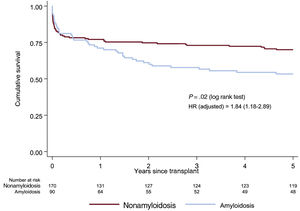
Restrictive and hypertrophic cardiomyopathy subgroups analysisTable 6 of the supplementary data summarizes the baseline characteristics of the amyloidotic and nonamyloidotic etiologies of RCM. Patients with amyloidosis were significantly older, had a higher body mass index, and less frequently underwent the bicaval technique than patients with other etiologies. In addition, patients with amyloidosis showed a nonsignificant tendency to be more frequently male, to have a lower percentage of peripheral vascular disease and prior sternotomy, and a higher percentage of anti-CD25. The survival rates for individuals with amyloidosis were lower than those with RCM due to other etiologies (figure 5). This significant association persisted even after multivariable adjustment (figure 5 and table 7 of the supplementary data).
Survival curves (Kaplan-Meier method) for 5-year mortality for amyloidosis and nonamyloidosis etiology of restrictive cardiomyopathy. Hazard ratio was adjusted for pulmonary vascular resistance, respiratory disease, mechanical ventilation, malignancy, cold ischemia time, surgical technique, transplant era, immunosuppressive induction.
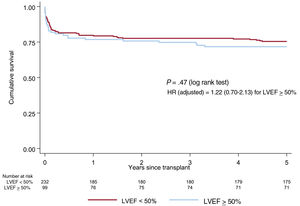
A high percentage of recipients with underlying HCM had an LVEF <50% at the time of HT (70%). These were more frequently male and received chronic amiodarone before HT compared with those without reduced ejection fraction at baseline. There were no between-group differences in either the univariable or adjusted survival analysis (figure 6, table 8 of the supplementary data).
Survival curves (Kaplan-Meier method) for 5-year mortality according to LVEF prior to heart transplant in recipients with hypertrophic cardiomyopathy. Hazard ratio was adjusted for age, Cytomegalovirus seropositivity, pulmonary vascular resistance, respiratory disease, mechanical ventilation, pretransplant sternotomy, urgency, transplant era and immunosuppressive induction. HR, hazard ratio; LVEF, left ventricular ejection fraction.
The results of the present analysis of a nationwide registry of HT can be summarized as follows (figure 7): a) HCM and RCM constitute a growing indication for HT; b) HCM has a higher 1-year mortality risk than DCM, and these results do not appear to have significantly improvement over the past 20 years. Nevertheless, the mid-term prognosis is similar to that of DCM; c) despite substantial enhancements in 1- and 5-year mortality risks over recent decades, the outlook for patients with RCM is less favorable. Among various restrictive conditions, cardiac amyloidosis has a notably unfavorable prognosis compared with other types of RCM; and d) there are no differences among the various LVEF phenotypes of HCM.
According to previous large registries, an increasing percentage of HCM and RCM patients have undergone HT.8,9 Notably, these trends are parallel with improvements in overall clinical status at both the time of listing and HT and correlate with improvements in overall survival.1,3,4,16,22 Furthermore, changes in allocation policies could also partially account for these findings, as is the case in the United States.5,6 In line with previous reports, we also observed significant differences in the characteristics of HCM and RCM recipients compared with DCM recipients. Patients with HCM and RCM were younger, were more likely to be female, and showed a tendency to have a lower prevalence of cardiovascular comorbidities than DCM patients,6,7 probably due to the predominantly genetic etiology of both cardiomyopathies. However, RCM cardiomyopathies constitute a more heterogeneous group in which the average clinical characteristics may vary depending on the relative contributions of the underlying etiology of the restrictive condition. For instance, a growing percentage of amyloidotic cardiomyopathy may result in an increase in the average age and a higher comorbidity burden of RCM recipients.14 Other between-group differences, such as those noted in donor characteristics and the use of the bicaval technique, should be understood within the context of broader temporal trends. This is particularly relevant since a significant proportion of the HCM and RCM cases in our study underwent HT during more recent periods.
In our study, the pretransplant clinical scenario did not seem to differ significantly for HCM and RCM compared with DCM. In fact, the presence of significant factors known to negatively influence early posttransplant prognosis tended to be less prevalent in the HCM/RCM groups. However, we cannot rule out the possibility that certain circumstances affecting relatively small groups, such as those patients using ventricular assist devices,16 or subtle differences that may be challenging to discern within the comprehensive description of a large registry, could have a significant impact on overall outcomes.
Like previous reports,9–12 our analysis showed worse early and total posttransplant survival in the RCM group compared with other etiologies, which persisted even after adjustment for potential confounders. It has been suggested that HT outcomes in patients with restrictive cardiomyopathy (RCM) could potentially be adversely influenced by factors such as reduced access to transplantation and worse medical status at the time of HT.4,26 Nonetheless, following the 2018 change in allocation policy by UNOS, there was no discernible difference in survival, despite the higher transplant rate and reduced waiting list duration.2,5 On the other hand, the limitations of mechanical circulatory support might also influence outcomes in allocation systems wherein prioritization on the waitlist is mainly based on the use of mechanical circulatory support. Nevertheless, these factors do not seem to influence the parity between patients with HCM/RCM and without HCM/RCM regarding the likelihood of receiving an HT.4 It is also possible that more granularity on unaccounted confounders such as the prevalence of previous malignancy, use of chest radiotherapy or cardiac toxic agents or the type and extent of cardiac amyloidosis might help to explain these findings.9,17 In amyloidosis, some reports suggest post-HT outcomes have improved in recent years27 due to a higher percentage of transthyretin amyloidosis (with less systemic involvement than amyloid light-chain amyloidosis), better candidate selection before HT, and improvements in the treatment of the primary disease before and after HT.16 However, consistent with previous findings, our analyses also demonstrate that amyloidosis continues to be associated with a higher risk of mortality than other etiologies of RCM.
We found that HCM and RCM share a similar early mortality rate, but significantly differ in terms of long-term survival, whereas survival in HCM equals that in DCM. Our results are fairly similar to those reported by Maron et al.21 from the OPTN/UNOS database analysis. These authors found that HCM patients demonstrated higher mortality than non-HCM patients after the initial first several months after HT but overall lower long-term mortality. Likewise, Zuñiga-Cisneros et al.7 reported a trend toward higher 1-year mortality in HCM patients compared with DCM patients before 2010. However, this early hazard was no longer seen in the more contemporary era. Contrary to our observations, these authors also noted an amelioration in the initial outcomes during more recent years, an improvement that we found only for long-term survival. As previously mentioned, lower age and the absence of systemic disease and/or malignancies could explain the improvement in long-term prognosis for HCM. However, the higher early mortality compared with DCM suggests the presence of common pretransplant clinical conditions and perioperative technical problems in HCM and RCM. We can speculate regarding the underlying causes of these observations by conducting a more in-depth analysis of the incidents and determinants of premature mortality within our study cohorts. First, both HCM and RCM exhibited a higher frequency of primary graft failure, a well-recognized prognostic factor, in comparison to DCM. This discrepancy might be associated with a more compromised clinical status preceding HT, a circumstance that remains challenging to fully comprehend given the constraints of the available data within our registry. Second, despite their limited representation in our dataset, the use of ventricular assist devices appears to exert an adverse impact on outcomes, which is particularly evident in the context of HCM. Consequently, within this specific subset of patients, the HR for 1-year mortality exhibited an escalation from 1.38 to 1.61. Third, an inclination toward prolonged cold ischemia times is discernible, a tendency likely attributed to procedural intricacies during the surgical intervention. Fourth, an excess in fatalities stemming from surgical complications in the HCM group, likely indicates more unfavorable conditions of the surgical field, alongside an excess of deaths resulting from causes differing from the typical antecedents of early mortality in RCM, a heterogeneous grouping presumably emblematic of the overall diminished physical condition stemming from the underlying disease. Of note, there were no differences in mortality according to the presence of reduced ejection fraction (LVEF <50%) and absence of reduced ejection fraction in HCM, suggesting this might not be a good marker of disease progression and severity.
LimitationsOur study presents an overview of a long-term nationwide registry in which we cannot exclude the potential presence of unaccounted-for confounding factors. A thorough characterization of pretransplant clinical status (time on waiting list, frailty markers, hemodynamic status), could be very useful in interpreting our findings. Although we had a modest amount of missing data, which were treated using standard techniques, the potential for data entry error persists. The relatively small sample size of some groups could have diminished the statistical power.
Inaccuracies in local adjudication of the primary etiology could have affected some cases. This could possibly explain the high prevalence of “idiopathic” or “other” causes of the restrictive phenotype. In addition, increased awareness of the diagnosis of certain cardiomyopathies, in particular amyloidosis, could explain its increasing prevalence as a cause of HT in recent years. Moreover, the database did not allow us to distinguish between different types of amyloidosis, and it does not consider the possibility of phenocopies in cases of hypertrophic/restrictive cardiomyopathies. Likewise, information on the presence and severity of intraventricular gradients in HMC is lacking. Our results should be interpreted in the context of the prolonged observation period, which entailed significant changes in the criteria for inclusion and care of patients on the waiting list as well as in posttransplant management.
CONCLUSIONSIn recent years, HCM and RCM have increasingly become indications for HT. The long-term outcomes following HT for HCM are comparable to those for more prevalent etiologies, such as DCM. However, in the case of RCM, particularly amyloidosis, we observed significantly worse short- and long-term prognosis post-HT compared with other etiologies. Our results indicate that there is significant room for improvement in terms of better recipient selection and perioperative management, primarily for amyloidosis and HCM.
FUNDINGThis study was carried out without financial support.
ETHICAL CONSIDERATIONSAll patients granted written informed consent upon their inclusion on the transplant waiting list, allowing the use of their deidentified data for research purposes. Additionally, the Spanish Registry of Heart Transplantation operations have received approval from the ethic committee of Hospital Universitario La Fe de Valencia (Spain). Sex and gender have been taken into account in the preparation of this article.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCEArtificial intelligence has not been used.
AUTHORS’ CONTRIBUTIONSAll authors meet the following 4 criteria: a) contributed substantially to the conception and design, acquisition of data, or its analysis and interpretation; b) drafted the article or critically reviewed it; c) gave final approval to the version to be published; d) agreed to take responsibility for the accuracy and integrity of the manuscript. I. Cruz and S. Lopes Fernandes contributed equally to this article.
CONFLICTS OF INTERESTThe authors declare that they have no conflict of interest.
- •
HCM and RCM are infrequent indications for HT.
- •
There is still some uncertainty regarding the posttransplant outcomes for recipients with underlying HCM and RCM.
- •
There are limited available data on the temporal trends of the outcomes of HT for these entities.
- •
A growing percentage of patients have been undergoing HT with a previous diagnosis of RCM and HCM (15% in the last 5 years, overall).
- •
Both RCM and HCM are associated with a lower short-term survival rate than DCM. However, this lower survival rate persists in the long-term for restrictive cardiomyopathy, but not for hypertrophic cardiomyopathy.
- •
Significant improvements in short-term posttransplant prognosis have been observed for RCM in the last 20 years.
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.rec.2023.10.006