Morbidity and mortality after admission for acute heart failure remain prohibitively high. In that setting, plasma levels of antigen carbohydrate 125 have shown to correlate with the severity of fluid overload and the risk of mortality and readmission. Preliminary data suggests a potential role of antigen carbohydrate 125 to guide therapy. The objective of this study is to evaluate the prognostic effect of an antigen carbohydrate 125-guided management strategy vs standard therapy in patients recently discharged for acute heart failure.
MethodsThis is a multicenter, randomized, single-blind, efficacy trial study of patients recently discharged from acute heart failure (< 180 days), New York Heart Association functional class II-IV and antigen carbohydrate 125 > 35 U/ml. A randomization scheme was used to allocate participants (in a 1:1 ratio) to receive therapy guided by antigen carbohydrate 125 (aiming to keep normal values) or standard treatment. Mainly, antigen carbohydrate 125-guided therapy is focused on the frequency of monitoring and titration of decongestive therapies and statins. As of December 10, 2013, there were 383 patients enrolled. The primary outcome was the composite of 1-year all-cause mortality or rehospitalization for acute heart failure. Analysis was planned to be intention-to-treat.
ConclusionsDiscovering novel therapeutic strategies or finding better ways of optimizing established treatments have become a health care priority in heart failure. This study will add important knowledge about the potential of antigen carbohydrate 125 as a management tool for monitoring and titration of therapies where optimal utilization has not been well defined, such as diuretics and statins.
Trial registration: ClinicalTrials.gov number: NCT02008110.
Keywords
After discharge from an acute heart failure (AHF) episode, even despite improvement in clinical status, rehospitalization and mortality rates remain excessively high during the first months.1–3 Fluid overload (FO) is a key feature in the pathogenesis of AHF,4,5 but its severity and distribution vary greatly between individual patients.4–7 Complicating this matter is the fact that many of these patients are discharged with residual congestion.4,5 Traditionally, FO has been assessed through symptoms and signs, despite their limited accuracy for its identification.5,8 Similarly, other tools such as chest X-ray, natriuretic peptides, echocardiography, and invasive measurements have shown poor performance for quantifying the severity of FO.5,8,9 Indeed, an accurate and clinically-driven method for quantifying the FO status remains an unmet need in the contemporary management of patients with AHF.
Tumor marker antigen carbohydrate 125 (CA-125), a glycoprotein widely used for ovarian cancer monitoring, has emerged as a potential biomarker of heart failure (HF).10 Plasma CA-125 correlates with clinical, hemodynamic, and echocardiographic parameters related to the severity of the disease.10,11 Particularly interesting is the correlation with symptoms and signs of FO and inflammatory markers.10 Indeed, high levels of this glycoprotein have shown to be present in most patients admitted for AHF12 and independently related to mortality and subsequent admission for AHF.10,12 Furthermore, certain characteristics such as wide availability, low-cost, and close correlation between plasma changes and clinical outcomes have increased the interest of researchers in the potential of this glycoprotein for monitoring and guiding therapy in HF.10–14 With respect to the latter point, preliminary evidence suggests that patients with elevated CA-125 levels would show greater clinical benefit from a more aggressive decongestive therapy.15 In addition, as a surrogate for heightened low-grade inflammation, high levels may also identify a subset of patients who benefit from statin treatment.16
Therefore, this study was designed to prove the hypothesis that a treatment strategy guided by plasma CA-125 would improve 1-year clinical outcomes compared to a usual care strategy in patients who have had a recent episode of AHF.
METHODSStudy DesignThis study was designed as a multicenter, randomized, single-blind, efficacy trial of patients recently discharged from an episode of AHF (< 180 days), with New York Heart Association functional class II-IV and CA-125 > 35 U/mL. A computer-generated randomization scheme was used to allocate participants (in a 1:1 ratio) to receive therapy guided by CA-125 (aiming to keep CA-125 ≤ 35 U/mL) or standard treatment. Patients (but not investigators) were masked to the group assignment. The study is being conducted in 5 centers in Spain. Independently of staggered entry, the minimum duration of a patient's participation is 12 months (from first to last visit). The study is being conducted in accordance with Good Clinical Practice, Declaration of Helsinki 2002. All patients provided signed informed consent and the protocol was approved by the research ethics committee of participating centers and Agencia Española de Medicamentos y Productos Sanitarios.
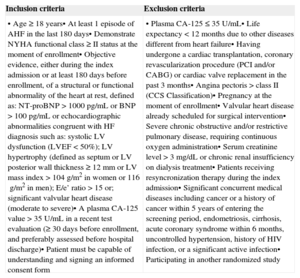
Study PopulationCandidate patients were selected from a consecutive cohort of patients with a recent admission for AHF. Inclusion and exclusion criteria are summarized in Table 1.
Inclusion and Exclusion Criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| • Age ≥18 years• At least 1 episode of AHF in the last 180 days• Demonstrate NYHA functional class ≥ II status at the moment of enrollment• Objective evidence, either during the index admission or at least 180 days before enrollment, of a structural or functional abnormality of the heart at rest, defined as: NT-proBNP>1000pg/mL or BNP>100pg/mL or echocardiographic abnormalities congruent with HF diagnosis such as: systolic LV dysfunction (LVEF < 50%); LV hypertrophy (defined as septum or LV posterior wall thickness ≥ 12mm or LV mass index > 104g/m2 in women or 116g/m2 in men); E/e’ ratio > 15 or; significant valvular heart disease (moderate to severe)• A plasma CA-125 value > 35 U/mL in a recent test evaluation (≥30 days before enrollment, and preferably assessed before hospital discharge)• Patient must be capable of understanding and signing an informed consent form | • Plasma CA-125 ≤ 35 U/mL• Life expectancy < 12 months due to other diseases different from heart failure• Having undergone a cardiac transplantation, coronary revascularization procedure (PCI and/or CABG) or cardiac valve replacement in the past 3 months• Angina pectoris > class II (CCS Classification)• Pregnancy at the moment of enrollment• Valvular heart disease already scheduled for surgical intervention• Severe chronic obstructive and/or restrictive pulmonary disease, requiring continuous oxygen administration• Serum creatinine level > 3 mg/dL or chronic renal insufficiency on dialysis treatment• Patients receiving resyncronization therapy during the index admission• Significant concurrent medical diseases including cancer or a history of cancer within 5 years of entering the screening period, endometriosis, cirrhosis, acute coronary syndrome within 6 months, uncontrolled hypertension, history of HIV infection, or a significant active infection• Participating in another randomized study |
AHF, acute heart failure; BNP, brain natriuretic peptide; CA-125, antigen carbohydrate 125; CABG, coronary artery bypass graft; CCS, Canadian Cardiovascular Society; HF, heart failure; HIV, human immunodeficiency virus; LV, left ventricular; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro-brain natriuretic peptide; NYHA, New York Heart Association; PCI, percutaneous coronary intervention.
Patient screening was conducted either as inpatient (before discharge from an AHF hospitalization) or as outpatient evaluation within the first 180 days after hospital discharge. After reviewing the inclusion/exclusion criteria and signing/dating the informed consent form, comprehensive medical history and physical examination were performed. All baseline tests and procedures were performed before treatment allocation.
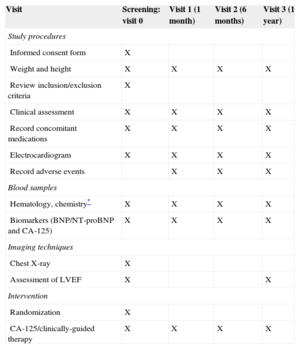
Follow-up VisitsAfter randomization, patients are evaluated at 1 month, 6 months, and 12 months (visits 1, 2 and 3, respectively). At each of these visits, a complete medical history, physical examination, 12-lead electrocardiogram, and blood samples for laboratory tests are obtained. Labs include hematology and chemistry, brain natriuretic peptides, and CA-125 serum levels, among others. The procedures scheduled for each visit are detailed in Table 2.
Follow-up Design
| Visit | Screening: visit 0 | Visit 1 (1 month) | Visit 2 (6 months) | Visit 3 (1 year) |
|---|---|---|---|---|
| Study procedures | ||||
| Informed consent form | X | |||
| Weight and height | X | X | X | X |
| Review inclusion/exclusion criteria | X | |||
| Clinical assessment | X | X | X | X |
| Record concomitant medications | X | X | X | X |
| Electrocardiogram | X | X | X | X |
| Record adverse events | X | X | X | |
| Blood samples | ||||
| Hematology, chemistry* | X | X | X | X |
| Biomarkers (BNP/NT-proBNP and CA-125) | X | X | X | X |
| Imaging techniques | ||||
| Chest X-ray | X | |||
| Assessment of LVEF | X | X | ||
| Intervention | ||||
| Randomization | X | |||
| CA-125/clinically-guided therapy | X | X | X | X |
BNP, brain natriuretic peptide; NT-proBNP, N-terminal pro-brain natriuretic peptide; CA-125, carbohydrate antigen 125; LVEF, left ventricular ejection fraction.
Optional visits are permitted irrespectively of the treatment group, if the physician in charge of the patients considers it clinically appropriate.
Study TreatmentPatients were randomly assigned to either the active or standard treatment group.
Standard treatment strategy:
- •
Patients randomized to this group are being treated according to contemporary European guidelines.17,18
- •
In patients with systolic dysfunction, standard care includes: a) angiotensin-converting enzyme inhibitors unless contraindicated; in case of intolerance, they should switch to angiotensin receptor blocker; b) beta-blockers at the maximum dose tolerated; c) aldosterone antagonists should be considered in patients with left ventricular ejection fraction (LVEF) ≤ 35%; d) ivabradine in patients with LVEF ≤ 35%, sinus rhythm, and heart rate ≥ 70 bpm; e) vasodilator therapy with hydralazine plus isosorbide dinitrate if angiotensin-converting enzyme inhibitors or angiotensin receptor blockers are contraindicated or poorly tolerated, and f) an angiotensin receptor blockers is recommended in patients with HF and LVEF≤40% who remain symptomatic despite optimal treatment with an angiotensin-converting enzyme inhibitor and beta-blocker, unless already taking an aldosterone antagonist.
- •
For patients with HF and preserved LVEF, treatment should be focused on: a) treating symptoms and signs of fluid retention with loop diuretics; b) encouraging the use of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, and c) indicating a beta-blocker or a calcium channel blocker (verapamil/diltiazem), in particular when blood pressure is > 130/80mmHg and/or the patient has atrial fibrillation.
- •
In all patients allocated to this arm, diuretic titration should be performed according to symptoms and signs of FO. Ambulatory intravenous loop diuretic administration may be indicated in cases with persistent or worsening symptoms and signs attributable to FO, despite administration of high oral loop diuretic doses.
- •
In all patients allocated to this arm, statins and omega-3 polyunsaturated fatty acids are not recommended, but are permitted.
- •
Other treatments (digoxin, nitrates, anticoagulants, antiarrhythmics, cardiac resynchronization therapy, cardiac revascularization, valvular heart surgery, heart transplantation and inotropes) are indicated in accordance with contemporary guidelines.17,18.
- •
Intravenous iron is recommended in patients with iron deficiency (serum ferritin level < 100¿g/L or between 100¿g/L and 299¿g/L when the transferrin saturation < 20%), hemoglobin level between 95¿g/L and 135¿g/L, and New York Heart Association functional class III-IV. Iron dose to be administered would be in accordance with the calculation of total iron deficit.
- •
Prescheduled visits were planned at 1 month, 6 months, and 12 months after randomization. Additional visits are scheduled in accordance with clinical status and at the discretion of the clinicians in charge of patients.
- •
CA-125 is measured in these patients, although the values are not available during the trial.
- •
Natriuretic peptides are measured in these patients and results are available during the trial, although no specific therapeutic recommendations are indicated according to their values.
CA-125 guided strategy:
- •
In this group, physicians are encouraged to maximize all treatment measures aimed to keep CA-125 ≤ 35 U/mL, while keeping the potential for side effects at a minimum. Overall, all patients should be treated following standard guidelines regarding angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, beta-blockers, aldosterone antagonists, ivabradine and other treatments, such as anticoagulants, antiarrhythmics, digoxin, nitrates and vasoactive group, devices, revascularization, and surgical procedures. Similarly, patients with HF and preserved LVEF should be managed following standard recommendations.17,18
- •
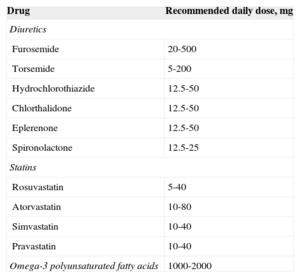
Overall, in this arm, the frequency of monitoring visits and titration of decongestive therapies, statins, intravenous iron, and omega-3 polyunsaturated fatty acids should be performed according to CA-125 evolution. The proposed algorithm is shown in Table 3. Recommended doses of drugs for the active arm are summarized in Table 4.
Table 3.Algorithm of Treatment (CA-125-guided Therapy)
Visit 0 • Consider use of statins in all patients, specially at low doses• Consider use of omega-3 polyunsaturated fatty acids• Maintain loop diuretic dosage if clinical stability. Consider increasing loop diuretic dosage if symptoms and signs of congestion persist Visits 1, 2, 3 and additional CA-125 returns to normal values (≤ 35 U/mL): • Consider reducing loop diuretic dose, especially in patients receiving high diuretic doses (furosemide equivalent dose ≥ 120 mg/day) and in those with evidence of worsening renal function• Encourage the initiation, if not prescribed, or the continuation of statin and omega-3 polyunsaturated fatty acids treatment if well tolerated CA-125 decreases but remains high (>35 U/ml): • Consider maintaining loop diuretic dose or increase dose if furosemide equivalent dose < 80 mg/day is currently prescribed• Reevaluate clinical status and CA-125 in an additional prompt visit (2-8 weeks)• Consider increasing statin dose• Consider prescribing omega-3 polyunsaturated fatty acids if previously not used• Consider up-titrating beta-blockers and/or ACE inhibitors and/or ARB doses to maximum doses recommended• Consider adding aldosterone antagonist if previously not administered• Consider administration of intravenous iron if iron deficiency is present CA-125 increases along the course of the trial: • Consider incrasing loop diuretic dose and/or adding hydrochlorothiazide 12.5-50 mg/day or clorthalidone 12.5-50 mg/day and/or aldosterone antagonist 12.5-50 mg/day• Consider optional prompt visits (1-4 weeks)• Consider ambulatory administration of intravenous furosemide and/or ultrafiltration techniques• Maximize the stating treatment if possible• Consider prescribing/increasing omega-3 polyunsaturated fatty acids• Consider intravenous iron if iron deficiency is present CA-125, antigen carbohydrate 125; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker.
Table 4.Recommended Doses of Diuretics, Stains and Omega-3 Polyunsaturated Fatty Acids for CA-125-guided Therapy Arm
Drug Recommended daily dose, mg Diuretics Furosemide 20-500 Torsemide 5-200 Hydrochlorothiazide 12.5-50 Chlorthalidone 12.5-50 Eplerenone 12.5-50 Spironolactone 12.5-25 Statins Rosuvastatin 5-40 Atorvastatin 10-80 Simvastatin 10-40 Pravastatin 10-40 Omega-3 polyunsaturated fatty acids 1000-2000 CA-125, antigen carbohydrate 125.
All end points are verified by an independent clinical monitor and subsequently submitted to an adjudication committee blinded to treatment assignments.
Primary End PointThe primary outcome is the composite of all-cause mortality or rehospitalization for AHF within 1 year after randomization (whichever occurred first and evaluating repeated events). Readmission for AHF was defined as an unplanned hospitalization requiring > 24-h stay and caused by substantive worsening of HF symptoms and/or signs requiring new administration of intravenous HF therapies, including inotropes, diuretics, or vasodilators.
Secondary End Points- •
Composite of all-cause mortality and readmission for any cause within 1 year after randomization.
- •
Days alive outside of the hospital at 1 year.
- •
Number of AHF hospitalizations at 1 year.
- •
Number of episodes of worsening HF not requiring hospitalization at 1 year.
- •
Percentage of patients who normalize CA-125 values (≤ 35 U/mL) and time required to achieve their goal.
- •
Effects on brain natriuretic peptide/N-terminal pro-brain natriuretic peptide levels (at 1 month, 6 months, and 12 months).
Based on the treatment strategies, the patient safety assessment is specifically focused on monitoring renal function, liver function, myopathy, and electrolyte disturbances.
Sample Size CalculationSample size determination for this study assumes 2-sided testing at the 0.05 significance alpha level. The effect size for the primary end point was based on prior results from the AHF registry at the Hospital Clínico Universitario (Valencia, Spain). In this pilot study, 1702 patients consecutively admitted to the hospital with a diagnosis of AHF were followed up for clinical end points. During the index hospitalization, 64.6% had CA-125 > 35 U/mL. For this registry, we have assumed that both CA-125 groups have received standard medical therapy, and thus the differences found in outcomes will not include the effect of an optimized therapy. A 1-year post-discharge rate for the composite end point of all-cause mortality or readmission for AHF was estimated as 42.8% for the group with CA-125 > 35 U/mL, and 32.6% for the group with CA-125 ≤ 35 U/mL, yielding an absolute rate difference of 10.4%. Therefore, we expect that optimizing treatment in the group with CA-125 > 35 U/mL will translate into 35% relative risk reduction of this combined end point. Thus, using 80% power to detect at least such difference at 1 year and a 1:1 allocation ratio, 180 patients were estimated for each group (n=360 patients). The total sample was increased to 378 patients (n=189 each group) in order to account for losses to follow-up, which was assumed to be around 5%.
Statistical Analysis PlanAll statistical comparisons will be made under the intention-to-treat principle. Continuous variables will be expressed as a mean (1 standard deviation) or median [interquartile range] as appropriate. Comparison of the both treatment strategies will be carried out with unpaired Student t test or Mann-Whitney U-test according to variable distribution. Discrete variables will be compared with chi-square test or Fisher exact test as appropriate.
We have envisioned 2 methodological approaches for the analysis of the primary end point:
- •
Analyzed as time to first event: In the first approach, which relies on the traditional way of analyzing results from clinical trials, the difference between the treatment groups will be graphically depicted by Kaplan-Meier method and tested by log rank test. The main censoring criteria will be the end of the trial (administrative censoring at 1 year) and time to first event. Univariate hazard ratios will be estimated with Cox proportional hazard regression; however, multivariable Cox regression analysis will be used only in the event that important prognostic factors or patient characteristics at baseline show significant imbalance between the two randomized groups. All analyses will account for a potential clustering effect within centers.
- •
Accounting for recurrent HF hospitalizations: To account for the patient's entire outcome history, we perform negative binomial regression analysis, where the count of events (including death, if it occurred) will be compared between the two treatment groups.
For the secondary end points, composite of death and any rehospitalization, a similar approach will be used. For the mortality endpoint, both groups will be compared using the joint modeling approach, which simultaneously adjusts for the longitudinal time-varying effect of CA-125 while accounting for death as a terminal event. For the end point of rehospitalization, the survival curve will be constructed as a cumulative incidence function, in order to account for mortality as a competing event. For these end points, however, we will also explore the use of joint frailty modeling to compare both groups in regard to recurrent hospitalizations along the entire follow-up, while adjusting for death as a terminal event. For the end point of days alive and out of the hospital, the length of the follow-up that will be used in the survival analysis will not include the length of time the patient has spent in the hospital on any admission.
Subgroup analyses will be performed among patients defined by age group (> 65 years, > 70 years, > 75 years), sex, statins use, LVEF (> 50%, > 40%), etiology, inflammatory status (relative lymphocyte count and high-sensitivity C-reactive protein), renal failure, CA-125 at baseline (above median), and natriuretic peptides at baseline (above median).
All analyses were planned to be intention-to-treat. A 2-sided P-value of < .05 is considered to be statistically significant for all analyses. All analyses will be performed with Stata 13.1 or the R package.
Current StatusThe protocol was approved by the ethics committee/institutional review board for each participating center. Patients started enrolling in December 2011. As of July 17, 2013, the study was fully enrolled. As of December 2013, >50% of patients had finished the follow-up.
DISCUSSIONMorbidity and Mortality Following an Episode of Acute Heart Failure: the Importance of Fluid Overload and Inflammatory StatusIn any national health system, AHF hospitalizations represent a health care burden1–3,19, being associated with prohibitively high morbidity and mortality rates soon after discharge.1–3 Paradoxically, most studies evaluating novel therapeutic strategies have been focused on stable HF patients and not on the AHF setting.17,18 It is our thinking that new research avenues need to be guided toward this vulnerable period in order to reduce the burden of this disease. In this regard, alternative strategies that seek to optimize decongestive therapies and control the inflammatory milieu seem to be pertinent goals, as both mechanisms play a critical role during this particular period.5,20 For instance, the severity of FO is related to poor prognosis5,21–23 and, far from being an epiphenomenon, it may promote the progression of the disease through complex neurohumoral (favoring sodium retention), renal (reducing glomerular filtration), cardiac, and endothelial interactions.24,25 Moreover, FO assessment and its treatment is frequently challenging and largely subjective, mainly because the clinical tools available, which include symptoms, signs, X-ray, and even natriuretic peptides, have shown limited accuracy in identifying or quantifying the degree of FO. This is particularly true for certain subgroups of patients carrying confounding conditions such as obesity, advanced age, and lung, liver, or peripheral vein diseases.5,8,9 Likewise, low-grade inflammation is common in patients with AHF, a fact that opens avenues for new treatment modalities such as statins and omega-3 polyunsaturated fatty acids,26,27 even though these treatments have failed to demonstrate substantial clinical benefits in previous studies.28,29
Based on the above background information, we designed this study in an effort to determine whether CA-125 values allow us to optimize treatment after an AHF episode.
Biomarker Guided Therapy in Heart FailureBiomarker guided therapy has recently emerged as an attractive strategy for personalizing the therapeutic options already available in HF management. Several studies have evaluated the clinical utility of natriuretic peptides-guided therapy in HF but, despite the slight improvement shown from pooled data,30,31 other relatively large studies have failed to document a significant clinical improvement in terms of mortality and morbidity.30–32 In fact, current US guidelines33 do not systematically recommend it for improving outcomes (class IIb); other clinical practice guidelines (including those from the European Society of Cardiology) are awaiting the results from new clinical trials. This lack of consensus is due, at least in part, to several uncertainties: a) determining which plasma level should be assumed as the optimal target level; this threshold is not well established because most studies have used different criteria; b) common confounders such as age, weight, renal function, and other comorbidities that have shown to influence the predictive ability of natriuretic peptides; c) very few large studies evaluating the clinical value of natriuretic-guided therapy in patients recently discharged for AHF, and d) logistic disadvantages such as price and high variability may also play a role.30–32 In our opinion, larger and better conducted trials addressing these unresolved issues should be undertaken in the future.
CA-125: Potential Biomarker for Monitoring and Guiding TherapyCA-125, also called MUC16, is a glycoprotein synthesized by epithelial serous cells, with extremely complex structure and high molecular weight. Although CA-125 is widely used to monitor ovarian cancer therapy, high plasma levels have also been reported in other malignant and nonmalignant diseases (HF, nephrotic syndrome, liver cirrhosis, tuberculosis, or pelvic inflammatory disease, among others).10 In the HF stetting, plasma CA-125 correlates with clinical (New York Heart Association functional class), hemodynamic (pulmonary artery wedge pressure, right atrial pressure), and echocardiographic (E wave deceleration time, right ventricular systolic dysfunction) parameters related to the severity of the disease.10,11 High levels are present in the majority of patients admitted for AHF; for instance, in a recent cohort of 1111 unselected patients admitted for AHF, 65% of patients displayed CA-125 > 35 U/mL.12 In this setting, CA-125 has been related to symptoms and signs of FO,10,12 inflammation status,34 and 6-month mortality and readmission,12 independently of natriuretic peptides and other traditional risk factors. In addition, recent studies10,12–16 have highlighted the potential of CA-125 to monitor and guide HF therapy in HF by exploiting certain characteristics, such as close correlation with disease severity and clinical outcomes, and as a marker that has the potential to differentially characterize the prognostic effect attributed to the use of loop diuretics doses and of statins. In fact, our group found that at the first outpatient visit after an admission for AHF (mean 28 days after discharge), those patients who normalized CA-125 levels exhibited the lowest adjusted risk of death, intermediate for those who had decreased but not normalized CA-125, and higher for those in whom CA-125 increased.13 These findings were also reproduced when predicting 6-month AHF readmission.14 More recently, Husser et al35 reported, in 228 patients with aortic stenosis who had undergone transcatheter aortic valve implantation, that the longitudinal evolution of CA-125 levels predicted adverse clinical outcomes after the procedure. Moreover, we reported in a cohort of patients admitted for AHF that discharge with high-dose loop diuretics was associated with higher risk of mortality, with the exception of a subgroup of patients characterized with elevated blood urea nitrogen and CA-125, in which the use of high-dose loop diuretics was associated with lower adjusted mortality rates.15 Furthermore, the use of statins has correlated with lower mortality rates in patients with AHF, but only when CA-125 was elevated.16
Other characteristics such as wide availability, low cost (around 2€ per determination), levels not substantially modified by age and renal dysfunction, and prolonged half-life (>1 week) make this biomarker a promising clinical tool in HF.10
CONCLUSIONsIn patients with AHF, plasma CA-125 correlates with FO, inflammatory status, and prognosis. Recently, this glycoprotein has emerged as a novel potential clinical tool for monitoring and titration therapy. In this trial we aim to evaluate, under controlled conditions, the role of CA-125 in optimizing the treatment of patients with a recent admission for AHF.
FUNDINGThis work is supported by a grant from Ministerio de Sanidad-España [Concesión de la ayudas para el fomento de la investigación clínica independiente. Orden SAS/2377/2010. EC10-108] and Instituto de Salud Carlos III, Red de Investigación Cardiovascular, Programa 7 (RD12/0042/0010 and RD/12/0042/0068) FEDER.
CONFLICTS OF INTERESTNone declared.
We thank the study coordinators, nurses, and staff at the investigative sites and especially all of the patients involved in this trial. Special acknowledgment to Marta Peiró, Gemma Romero, Anna Mollar, Estefania Montalvo and INCLIVA (Fundación para la Investigación del Hospital Clínico de la Comunidad Valenciana) workers for the logistic and institutional support.
Hospital Clínico Universitario, Valencia, Spain: Julio Núñez, Sergio García-Blas, Juan Sanchis, Vicent Bodí, Enrique Santas, Miryam Olivares, Clara Bonanad, Lourdes Bondanza, and Francisco J. Chorro.
Hospital de la Plana, Castellón, Spain: Maria José Bosch, Pilar Merlos, Jorge Gallego, and Patricia Palau.
Hospital de Manises, Manises, Valencia, Spain: Pau Llàcer, Andrea Mendizábal, Gema Miñana, Valle Pedrosa, Montserrat Salvador, Antonio Camps, and Gonzalo Salvador.
Hospital de San Juan, San Juan de Alicante, Alicante, Spain: Vicente Bertomeu-González, Vicente Bertomeu-Martínez, Alberto Cordero, José Moreno, Juan Quiles, and Adriana López Pineda.
Hospital General Universitario, Valencia, Spain: Lorenzo Fácila, Vicente Montagud, Rosa Fonfria, Maria Teresa Jareño, Joaquina Belchi, Eva Rumiz, and Salvador Morell.