To the Editor,
Transcatheter implantation of aortic valve prostheses is being performed with increasing frequency in patients with severe symptomatic aortic stenosis who are at high surgical risk. Either a transfemoral or a transapical approach is employed. Complications related to the procedure are relatively uncommon, but they provide information that is very useful for broadening our knowledge of the pathophysiology of prosthesis dysfunction.
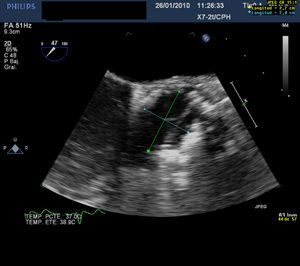
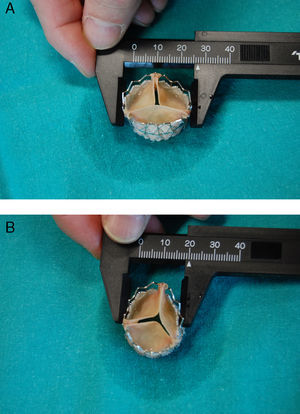
A 76-year-old man with severe aortic stenosis underwent transcatheter aortic valve implantation because of high surgical risk due to ischemic heart disease and severe chronic obstructive pulmonary disease. Intraoperative transesophageal echocardiogram (TEE) performed prior to the procedure revealed a severely calcified aortic valve, especially left coronary (Thebesian) valve; the ejection fraction was 42%. Balloon valvuloplasty was carried out, followed by implantation of a 26-mm SAPIEN valve (Edwards Lifesciences; Irvine, California, USA), performed without complications. Immediately after inflation, TEE confirmed that the aortic prosthesis was well positioned, with adequate valve mobility. However, probably due to the severe eccentric calcification, the prosthesis had an asymmetric morphology, with an oval shape and abnormal stretching of the valve, which was oriented along the major axis (Figure 1). Despite this appearance, the results of the procedure were considered to be satisfactory because the prosthesis appeared to be functioning normally, with mild central and minimal paravalvular regurgitation. Initially, the patient progressed well and was extubated on the first day; however, the next day he developed acute pulmonary edema, with rapid clinical deterioration. An emergency echocardiogram revealed severe aortic regurgitation, and the patient underwent an emergency intervention involving extracorporeal surgery with implantation of a Perimount bioprosthesis (Edwards Lifesciences; Irvine, California, USA). The patient died of cardiogenic shock during the postoperative period. Visual inspection of the explanted SAPIEN valve showed an elliptical morphology with a major diameter of 27mm and a minor diameter of 20mm, measurements that agree with those made by means of TEE during the procedure (Figure 2). Moreover, as documented with TEE, one of the valves was abnormally taut and elongated, with limited mobility.
Figure 1. Transesophageal echocardiogram immediately after prosthesis implantation. The measurements made from short axis views of aorta show a major diameter of 27mm and a minor diameter of 20mm, in agreement with the measurements made in the explanted prosthesis. Also note the asymmetrical appearance of the valves.
Figure 2. Measurements of the major diameter (A) and minor diameter (B) of the explanted prosthesis. Also note the asymmetrical appearance of the valves.
The Edwards-SAPIEN valve is a prosthesis made of bovine pericardium mounted on an expandable stent that is placed in subcoronary position.1 Nine years after the first case in humans,2 favorable results have been reported for both the transfemoral and the transapical approach.3, 4, 5 The complete and symmetric expansion of the prosthesis in the aortic annulus is very important for its normal function and the aim should be to achieve this in every case. In fact, when the valve has a circular aspect, a success rate of 98% can be expected, whereas an oval morphology is associated with suboptimal function and durability.
In our case, the massive presence of eccentric calcium in the left coronary valve of the native valve presumably provoked abnormal stress in the anteroposterior direction and impeded uniform circular expansion. In fact, the major diameter of the prosthesis was greater than the nominal diameter, which indicates that the problem was not an insufficient inflation pressure, but the lack of deformability of the annulus in a given direction. The consequence was an abnormal tautness in the valve oriented along the major axis, which resulted in limited mobility, inadequate coaptation, and finally, severe aortic regurgitation. Although this aortic insufficiency was considered to be mild at the end of the procedure because of the narrow width of the jet, the abnormal geometry of the prosthesis may have been what caused the progression to severe regurgitation during the postoperative period.
Severe asymmetric valve calcification is a risk factor for incomplete expansion of the prosthesis and requires special attention to technique, even balloon oversizing. The failure to achieve adequate expansion can lead to severe aortic regurgitation that could have a negative influence on the postoperative course. We propose that, when the prosthesis is seen to have an asymmetric morphology during the procedure, the echocardiographic examination should include measurement of the major diameter. If it is greater than the nominal diameter and there is central regurgitation, regardless of the severity, the balloon should be reinflated and, if this proves to be ineffective, a “valve-in-valve” procedure should even be considered to increase the radial strength. In any case, close observation with serial echocardiograms will be necessary to enable the early detection of functional deterioration in the prosthesis and the need for therapeutic intervention.
.
Corresponding author: mcavero.hpth@salud.madrid.org