Recent advances have linked increased oxidative stress to a direct decrease in myocardial function.1 One indirect marker of oxidative stress is uric acid (UA). Increased serum concentrations of UA have a predictive value for adverse events in patients with heart failure (HF) and are associated with a worse hemodynamic profile.2,3 Moreover, in patients with HF and elevated UA, treatment of the hyperuricemia has clinical benefits that correlate with the degree of UA reduction.4 However, no studies have confirmed the association between serum UA concentrations and worse prognosis in patients with acute HF.
We retrospectively evaluated the short- and long-term prognostic implications of the serum UA concentration in patients older than 50 years admitted to our internal medicine department for acute decompensated HF, both de novo and acute chronic HF (according to the criteria of the European Society of Cardiology). The patients’ demographic data, cardiovascular history, functional capacity for activities of daily living via the Barthel index, Charlson comorbidity index, and New York Heart Association functional class were recorded using the institutional information system and electronic medical records. Also collected were the patients’ clinical and laboratory values, treatments, and echocardiography findings at admission and patient mortality during the index admission and at the end of a 1-year follow-up period. Subsequently, after the exclusion of patients with end-stage disease in palliative care, patients with acute HF and hyperuricemia at admission were compared with those with normal UA concentrations. The UA was measured in the first 48hours of emergency department admission; men and women were considered hyperuricemic with plasma concentrations of > 8mg/dL and > 7mg/dL, respectively. Quantitative variables are shown as means ± standard deviation and categorical variables as percentages. The characteristics of the 2 groups (hyperuricemic vs normouricemic) were compared using chi-square and Student t tests. Logistic regression analysis and multivariable Cox proportional hazard risk analysis were used to evaluate the in-hospital and 1-year mortality rates. Statistical significance was defined as P < .05.
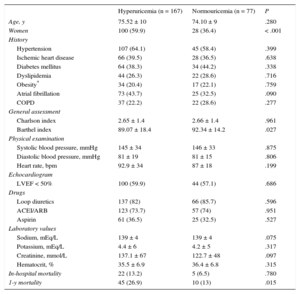
A total of 244 consecutive patients admitted for acute HF were evaluated, 128 women (52.5%) and 116 men, with a mean age of 75 ± 10 years and moderate comorbidity (Charlson index of 2.6). In total, 144 (59%) showed a preserved left ventricular ejection fraction (> 50%); 203 (83.1%) were receiving diuretics, and 180 (73.7%) patients were using angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers. A considerable majority—167 of the 244 patients (68.4%)—had hyperuricemia at admission, mainly women, and these hyperuricemic patients had a higher degree of functional dependence and higher serum creatinine concentrations than normouricemic patients with acute HF. Normouricemic patients showed a greater tendency for diabetes mellitus and obesity (Table).
Clinical Characteristics and Laboratory Results of Acute Heart Failure Patients With and Without Hyperuricemia
| Hyperuricemia (n = 167) | Normouricemia (n = 77) | P | |
|---|---|---|---|
| Age, y | 75.52 ± 10 | 74.10 ± 9 | .280 |
| Women | 100 (59.9) | 28 (36.4) | < .001 |
| History | |||
| Hypertension | 107 (64.1) | 45 (58.4) | .399 |
| Ischemic heart disease | 66 (39.5) | 28 (36.5) | .638 |
| Diabetes mellitus | 64 (38.3) | 34 (44.2) | .338 |
| Dyslipidemia | 44 (26.3) | 22 (28.6) | .716 |
| Obesity* | 34 (20.4) | 17 (22.1) | .759 |
| Atrial fibrillation | 73 (43.7) | 25 (32.5) | .090 |
| COPD | 37 (22.2) | 22 (28.6) | .277 |
| General assessment | |||
| Charlson index | 2.65 ± 1.4 | 2.66 ± 1.4 | .961 |
| Barthel index | 89.07 ± 18.4 | 92.34 ± 14.2 | .027 |
| Physical examination | |||
| Systolic blood pressure, mmHg | 145 ± 34 | 146 ± 33 | .875 |
| Diastolic blood pressure, mmHg | 81 ± 19 | 81 ± 15 | .806 |
| Heart rate, bpm | 92.9 ± 34 | 87 ± 18 | .199 |
| Echocardiogram | |||
| LVEF < 50% | 100 (59.9) | 44 (57.1) | .686 |
| Drugs | |||
| Loop diuretics | 137 (82) | 66 (85.7) | .596 |
| ACEI/ARB | 123 (73.7) | 57 (74) | .951 |
| Aspirin | 61 (36.5) | 25 (32.5) | .527 |
| Laboratory values | |||
| Sodium, mEq/L | 139 ± 4 | 139 ± 4 | .075 |
| Potassium, mEq/L | 4.4 ± 6 | 4.2 ± 5 | .317 |
| Creatinine, mmol/L | 137.1 ± 67 | 122.7 ± 48 | .097 |
| Hematocrit, % | 35.5 ± 6.9 | 36.4 ± 6.8 | .315 |
| In-hospital mortality | 22 (13.2) | 5 (6.5) | .780 |
| 1-y mortality | 45 (26.9) | 10 (13) | .015 |
ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin II receptor blockers; COPD, chronic obstructive pulmonary disease; HF, heart failure; LVEF, left ventricular ejection fraction.
Values are expressed as no. (%) or mean ± standard deviation.
During the index admission, 27 patients (11.1%) died, with 28 more patients dying during the first year, giving a cumulative 1-year mortality rate of 22.4%. There was no difference in the mortality rate during the index admission between acute HF patients with and without hyperuricemia (13.2% vs 6.5%; P = .780); however, 1-year mortality was significantly higher in patients with acute HF and hyperuricemia (26.9% vs 13.0%; P = .015). After the Cox regression analysis, high UA concentrations were still associated with 1-year mortality (hazard ratio [HR] = 1.091; 95% confidence interval [95%CI], 1.018-1.169) and the results indicated a protective effect of a better Barthel index at admission (HR = 0.979; 95%CI, 0.969-0.989).
Thus, hyperuricemia was a common finding in our patients requiring hospital admission for acute HF, although its cause is probably multifactorial, either due to the diuretic treatment received, its frequent association with renal dysfunction, or xanthine oxidase overexpression due to the proinflammatory status.4,5 In line with the few available studies, our results show higher long-term risk of death for patients admitted for acute HF with hyperuricemia, although not of in-hospital death during the index admission. However, there is currently no consensus on whether hyperuricemia plays a direct pathogenic role in the myocardium or whether it acts as a simple surrogate marker of severity.
One question to consider is if the mere reduction in UA can be beneficial in patients with HF. Further work evaluating the effect of hyperuricemic treatment of these patients is required.6