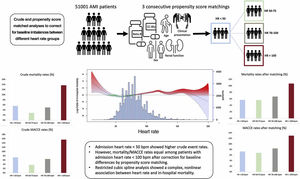
The risk prediction scores adopted in acute coronary syndromes (ACS) use incremental models to estimate mortality for heart rate (HR) above 60 bpm. Nonetheless, previous studies reported a nonlinear relationship between HR and events, suggesting that low HR may have an unrecognized prognostic role. We aimed to assess the prognostic impact of low HR in ACS, defined as admission HR <50 bpm.
MethodsThis study analyzed data from the AMIS Plus registry, a cohort of hospitalized patients with ACS between 1999 and 2021. The primary endpoint was in-hospital all-cause mortality, while a composite of all-cause mortality, major cardiac/cerebrovascular events was set as the secondary endpoint. A multilevel statistical method was used to assess the prognostic role of low HR in ACS.
ResultsThe study included 51 001 patients. Crude estimates showed a bimodal distribution of primary and secondary endpoints with peaks at low and high HR. A nonlinear relationship between HR and in-hospital mortality was observed on restricted cubic spline analysis. An HR of 50 to 75 bpm showed lower mortality than HR <50 bpm (OR, 0.67; 95%CI, 0.47-0.99) only after primary multivariable analysis, which was not confirmed after multiple sensitivity analyses. After propensity score matching, progressive fading of the prognostic role of HR <50 bpm was evident.
ConclusionsLow admission HR in ACS is associated with a higher crude rate of adverse events. Nonetheless, after correction for baseline differences, the prognostic role of low HR was not confirmed. Therefore, low HR probably represents a marker of underlying morbidity. These results may be clinically relevant in improving the accuracy of risk scores in ACS.
Keywords
Resting heart rate (HR) is an easy to measure, readily available, inexpensive physiological parameter measurable in all clinical conditions. Decades of research have evaluated the prognostic value of resting HR in different clinical conditions along with its role as a cardiovascular and metabolic risk factor. Its relevance has been confirmed both among healthy subjects and in patients with known cardiovascular diseases such as hypertension, metabolic syndrome, and diabetes.1 The deleterious effect of increased HR has been also recognized as a factor known to promote the development and progression of coronary artery disease.2
In patients with acute chest pain and clinical suspicion of an acute coronary syndrome (ACS), HR at admission is the very first clinical sign recorded at presentation, often before any other clinical or laboratory parameter such as blood pressure, ECG, or cardiac biomarkers.
Due to its easy, fast, and reliable assessment, its obvious clinical usefulness and the continuous nature of this variable, HR has been recognized as one of a few factors able to stratify prognosis of ACS patients at admission and is thus included in several clinical risk prediction scores such as PURSUIT and GRACE.3,4 The importance of these scores in clinical practice has been stressed by current European guidelines suggesting that, beside clinical assessment, use of the GRACE risk score is able to predict short- and long-term outcomes in ACS patients.5
While GRACE adopted an incremental model to account for the detrimental prognostic impact of HR in ACS with a progressive increase in the risk of in-hospital and 6-month mortality for HR only above 60 beats per minute, assuming that patients with HR <50 bpm have the same risk as those with a HR of 60 bpm,4,6 some evidence suggested a nonlinear, relationship between HR and event rates, with high event rates evident also for low HRs.
In particular, numerous retrospective studies describing the relationship between adverse events and admission HR reported the pattern known as the “J-shaped curve”, with higher rates at both extremities of HR distribution (ie, very low and high HR).7–11 This finding has also been confirmed by a large post hoc analysis of the CRUSADE quality improvement initiative including data from 135 164 patients.12
Nonetheless, the association between bradycardia and an increased likelihood of short-term events remains elusive and potentially biased. This is because, even in large ACS cohorts, a definite association could not be ultimately confirmed due to the relatively small sample size of patients presenting with low HR at admission,7,9,11 the lack of relevant data on associated atrioventricular conduction disorders,11 and the presence of critical baseline differences in the absence of adequate corrections adopted to balance the effect of clinical characteristics with renewed prognostic impact among different HR groups.
Thus, the aim of our study was to evaluate the association between bradycardia at admission and clinical outcomes in myocardial infarction (MI) in a large cohort of contemporary patients enrolled in the multicenter, nationwide Acute Myocardial Infarction in Switzerland (AMIS) Plus registry.
METHODSStudy design and objectiveThe AMIS Plus is an investigator initiated, nationwide, prospective registry collecting clinical data and investigator-reported outcomes of patients admitted with MI (with or without ST segment elevation) in more than 84 Swiss hospitals with currently ongoing enrolment since 1997. Details on the registry are available online.13 Participating centers provide data for each patient on a voluntary basis by a dedicated standardized questionnaire including over 300 items.
Data collection is centralized at the Epidemiology, Biostatistics and Prevention institute of the University of Zurich and checked for consistency. The registry was approved by the Swiss Federal Ethics Committee for Clinical Studies, the Swiss Board for Data Security, and the appropriate Cantonal Ethics Commissions. The present analysis was authorized by the AMIS Plus Steering Committee. The study protocol adheres to the ethics guidelines of the Declaration of Helsinki.
The objective of this registry-based study was to assess the correlation between HR <50 bpm at admission and short-term mortality in patients with MI.
Study populationThe study included patients enrolled in the registry with either ST- or non–ST-elevation MI (STEMI, NSTEMI) between January 1, 1999 and May 27, 2021. MI was defined according to the Universal Definition of Myocardial Infarction at the time of the patient's enrolment.14–17
Exclusion criteria were missing data on HR at admission, evidence of atrioventricular (AV) block with grade ≥ 2, wide QRS tachycardia, paced rhythm, or other unspecified rhythms at admission.
To identify the clinical and prognostic impact of different HR at presentation, to provide a clinically meaningful message, to allow comparisons with recent registry derived analyses7 and to ultimately verify whether the assumption that estimated risk for patients with HR at admission <50 bpm is equal to those of patients with HR 60 bpm, we stratified patients into 4 different groups according to HR at the time of first ECG recorded (HR <50, 50-75, 76-100 and> 100 bpm).
OutcomesThe primary endpoint was set as in-hospital all-cause mortality. A composite including all-cause mortality and major cardiac and cerebrovascular events (MACCE) such as cerebrovascular events and recurrent infarctions was set as a secondary endpoint.
Among all in-hospital complications collected, those considered in the present analysis were defined as:
- •
Cardiogenic shock: persistent signs of hypotension and/or pulmonary edema (Killip class IV) along with clinical or laboratory signs of impaired cardiac output with tissue hypoperfusion.
- •
Cerebrovascular event: any neurological event, whether ischemic, thrombotic, or hemorrhagic, confirmed by a neurologist and/or on brain imaging.
- •
In-hospital recurrent infarction: clinical signs or symptoms of ischemia with ECG changes indicative of new ischemia (new ST-changes or new left bundle branch block) and a newly identified increment of cardiac biomarkers (defined as a 2-fold increase in either creatine kinase MB fraction above the upper limit of normal or troponin above individual hospital cutoff levels for MI) following the initial clinical event.
Data are presented as the percentage of valid cases for categorical variables and as mean±standard deviation and/or medians [interquartile range] for continuous variables. Differences in baseline characteristics were compared using the unpaired t test or Kruskal-Wallis rank sum test, if appropriate, and the Pearson chi-square test.
Statistics are based on all cases with valid data for the respective variable. To ultimately verify the association between bradycardia and prognosis in patients with MI, a multilevel statistical approach was adopted. The association between clinical parameters including HR and in-hospital mortality was assessed adopting a traditional approach replicating those used in previously published analyses,7–11 based on univariate and multivariable analyses. A logistic regression stepwise approach was thus adopted to identify predictors of in-hospital mortality in the whole population. To test the robustness of the association between different HR groups and outcome, multiple sensitivity multivariate logistic regression analyses were performed in different patient subgroups alternatively including/excluding participants with ongoing therapies potentially impacting on HR at admission (namely beta-blockers and nondihydropyridine calcium channel blockers), patients with atrial fibrillation and finally adopting a random effect for study center.
The logistic regression analyses were performed using all cases with complete data for the considered variables.
An additional sensitivity analysis was also conducted to evaluate the relationship between admission HR (considered as a continuous variable) and the risk of in-hospital mortality using logistic regression and modelling HR with restricted cubic splines.18 A generalized additive model with penalized regression splines was used to fit the model. This resulted in 12.47 degrees of freedom.
We also conducted 3 sequential propensity score matched analyses to correct for baseline imbalances between different HR groups (namely HR <50 vs HR 50-75 bpm, HR <50 vs HR 76-100 bpm and HR <50 vs HR> 100 bpm).
To estimate propensity scores, the following 8 clinically relevant characteristics were included in a logistic regression with 1 of the dichotomous HR group variables as the dependent variable: age, sex, creatinine <83μmol/L (dichotomized at median), resuscitated out-of-hospital cardiac arrest (OHCA), cardiogenic shock at admission, STEMI, regular use of beta-blockers or calcium channel blockers. A 1:1 matching was performed. If there were missing values for the above-mentioned variables, data were replaced by multiple imputations using chained equations with 10 iterations and 5 datasets generated.
The statistical efficacy of the procedure was assessed by comparing standardized mean differences between matched patients among the 3 samples. The results of logistic regression are reported as odds ratios (OR) with a 95% confidence interval (95%CI). A probability value of P <.05 was considered significant. The IBM SPSS Statistics Version 27 (Armonk, IBM Corp, United States) and R statistics (Version 4.0.5) were used for the statistical analyses.
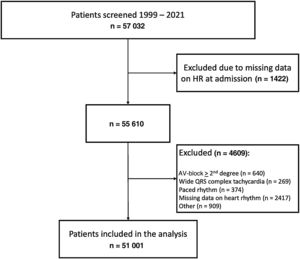
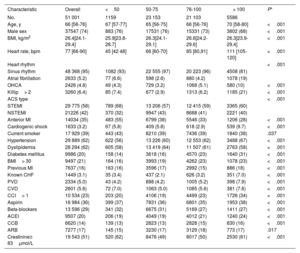
RESULTSOut of 59 354 patients enrolled in the registry in the predefined study period, 51 001 (85%), with a median age of 66 years [interquartile range, 56-76 years], fulfilled study inclusion criteria. Figure 1 shows patients’ flow. Baseline clinical characteristics stratified according to HR at admission are reported in table 1. reports the acute treatments offered at admission.
Baseline characteristics stratified by admission HR.
| Characteristic | Overall | <50 | 50-75 | 76-100 | > 100 | P* |
|---|---|---|---|---|---|---|
| No. | 51 001 | 1159 | 23 153 | 21 103 | 5586 | |
| Age, y | 66 [56-76] | 67 [57-77] | 65 [56-75] | 66 [56-76] | 70 [58-80] | <.001 |
| Male sex | 37547 (74) | 883 (76) | 17531 (76) | 15331 (73) | 3802 (68) | <.001 |
| BMI, kg/m2 | 26.4[24.1-29.4] | 25.9[23.8-28.7] | 26.3[24.1-29.1] | 26.6[24.2-29.6] | 26.3[23.9-29.4] | <.001 |
| Heart rate, bpm | 77 [66-90] | 45 [42 48] | 66 [60-70] | 85 [80,91] | 111 [105-120] | <.001 |
| Heart rhythm | <.001 | |||||
| Sinus rhythm | 48 368 (95) | 1082 (93) | 22 555 (97) | 20 223 (96) | 4508 (81) | |
| Atrial fibrillation | 2633 (5.2) | 77 (6.6) | 598 (2.6) | 880 (4.2) | 1078 (19) | |
| OHCA | 2426 (4.8) | 49 (4.3) | 729 (3.2) | 1068 (5.1) | 580 (10) | <.001 |
| Killip> 2 | 3260 (6.4) | 85 (7.4) | 677 (2.9) | 1313 (6.2) | 1185 (21) | <.001 |
| ACS type | <.001 | |||||
| STEMI | 29 775 (58) | 789 (68) | 13 206 (57) | 12 415 (59) | 3365 (60) | |
| NSTEMI | 21226 (42) | 370 (32) | 9947 (43) | 8688 (41) | 2221 (40) | |
| Anterior MI | 14034 (35) | 483 (55) | 6799 (38) | 5546 (33) | 1206 (28) | <.001 |
| Cardiogenic shock | 1633 (3.2) | 67 (5.8) | 409 (5.8) | 618 (2.9) | 539 (9.7) | <.001 |
| Current smoker | 17 929 (39) | 443 (43) | 8210 (39) | 7436 (39) | 1840 (38) | .037 |
| Hypertension | 29 889 (62) | 622 (56) | 13 226 (60) | 12 553 (62) | 3488 (67) | <.001 |
| Dyslipidemia | 28 294 (62) | 605 (58) | 13 419 (64) | 11 507 (61) | 2763 (58) | <.001 |
| Diabetes mellitus | 9986 (20) | 158 (14) | 3618 (16) | 4570 (23) | 1640 (31) | <.001 |
| BMI> 30 | 9497 (21) | 164 (16) | 3993 (19) | 4262 (23) | 1078 (23) | <.001 |
| Previous MI | 7637 (16) | 163 (16) | 3596 (17) | 2992 (15) | 886 (18) | <.001 |
| Known CHF | 1449 (3.1) | 35 (3.4) | 437 (2.1) | 626 (3.2) | 351 (7.0) | <.001 |
| PVD | 2334 (5.0) | 43 (4.2) | 888 (4.2) | 1005 (5.2) | 398 (7.9) | <.001 |
| CVD | 2601 (5.6) | 72 (7.0) | 1063 (5.0) | 1085 (5.6) | 381 (7.6) | <.001 |
| CCI> 1 | 10 534 (23) | 203 (20) | 4106 (19) | 4499 (23) | 1726 (34) | <.001 |
| Aspirin | 16 984 (36) | 399 (37) | 7831 (36) | 6801 (35) | 1953 (38) | <.001 |
| Beta-blockers | 13 596 (29) | 341 (32) | 6675 (31) | 5169 (27) | 1411 (27) | <.001 |
| ACEI | 9507 (20) | 206 (19) | 4049 (19) | 4012 (21) | 1240 (24) | <.001 |
| CCB | 6620 (14) | 139 (13) | 2823 (13) | 2828 (15) | 830 (16) | <.001 |
| ARB | 7277 (17) | 145 (15) | 3230 (17) | 3129 (18) | 773 (17) | .017 |
| Creatinine≥ 83μmol/L | 19 543 (51) | 520 (62) | 8476 (49) | 8017 (50) | 2530 (61) | <.001 |
ACEI, angiotensin converting enzyme inhibitors; ACS, acute coronary syndrome; ARB, angiotensin receptor blocker; BMI, body mass index; CAD, coronary artery disease; CCB, calcium channel blockers; CCI, Charlson comorbidity index; CHF, congestive heart failure; CVD, cardiovascular disease; MI, myocardial infarction; NSTEMI, non–ST-segment elevation myocardial infarction; OHCA, out-of-hospital cardiac arrest; PVD, peripheral vascular disease; STEMI, ST-segment elevation myocardial infarction.
Values are reported as No. (%) or median [interquartile range]; *Kruskal-Wallis rank sum test; Pearson chi-square test.
HR groups differed in several clinically relevant baseline characteristics with a known impact on short-term adverse event rates, including rates of resuscitated OHCA, cardiogenic shock, and presentation with STEMI vs NSTEMI.
Overall, 2525 in-hospital deaths were observed, leading to a crude in-hospital mortality of 5.0%. Crude estimates of in-hospital mortality were significantly different among HR groups, replicating the so-called “J shape curve”, with a bimodal distribution of events showing higher rates at very low and high admission HR.
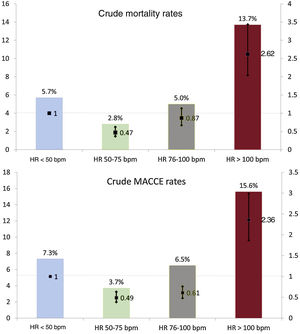
In patients with HR <50 bpm, 66 (5.7%) deaths were observed (reference), while 642 (2.8%; OR, 0.47; 95%CI, 0.37-0.62; P <.001) were observed in patients with HR 50 to 75 bpm, 1053 (5.0%) in patients with HR, 76 to 100 bpm (OR, 0.87; 95%CI, 0.67-1.13; P=.290), and 764 (13.7%) in patients with HR> 100 bpm (OR, 2.62; 95%CI, 2.04-3.43; P <.001).
Overall, the secondary composite endpoint occurred in 3157 patients, with significant differences throughout the HR distribution (P <.001 among groups). Rates of the individual components of the composite endpoint showed significant differences among HR groups. Mortality and MACCE rates along with associated OR stratified by HR group are reported in figure 2. reports additional details regarding in-hospital outcomes.
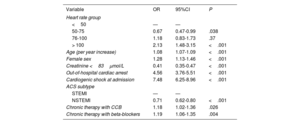
After multivariable logistic regression analysis, admission HR 50 to 75 was recognized as independently associated with lower in-hospital mortality compared with HR <50bpm (OR, 0.67; 95%CI, 0.47-0.99 P=.038) while HR> 100bpm was associated with a further increase in mortality risk (OR, 2.13; 95%CI, 1.48-3.15 P <.001). Additional independent predictors of increased in-hospital mortality were age, female sex, impaired renal function, OHCA, cardiogenic shock at admission, STEMI presentation and chronic therapy with either beta-blockers or calcium channel blockers. Table 2 reports the results of the primary multivariable regression analysis.
Multivariable logistic regression analysis: independent clinical parameters associated with in-hospital mortality.
| Variable | OR | 95%CI | P |
|---|---|---|---|
| Heart rate group | |||
| <50 | — | — | |
| 50-75 | 0.67 | 0.47-0.99 | .038 |
| 76-100 | 1.18 | 0.83-1.73 | .37 |
| > 100 | 2.13 | 1.48-3.15 | <.001 |
| Age (per year increase) | 1.08 | 1.07-1.09 | <.001 |
| Female sex | 1.28 | 1.13-1.46 | <.001 |
| Creatinine <83μmol/L | 0.41 | 0.35-0.47 | <.001 |
| Out-of-hospital cardiac arrest | 4.56 | 3.76-5.51 | <.001 |
| Cardiogenic shock at admission | 7.48 | 6.25-8.96 | <.001 |
| ACS subtype | |||
| STEMI | — | — | |
| NSTEMI | 0.71 | 0.62-0.80 | <.001 |
| Chronic therapy with CCB | 1.18 | 1.02-1.36 | .026 |
| Chronic therapy with beta-blockers | 1.19 | 1.06-1.35 | .004 |
95%CI, 95% confidence interval; ACS, acute coronary syndrome; CCB, calcium channel blockers; NSTEMI, non–ST-elevation myocardial infarction; OR, odds ratio; STEMI, ST-elevation myocardial infarction.
Nonetheless, multiple sensitivity multivariable logistic regression analyses performed after exclusion of patients on regular therapy with either beta-blockers or calcium channel blockers or adopting a random effect for study center did not confirm the protective effect of HR 50 to 75 bpm, which was borderline significant after exclusion of patients with atrial fibrillation for in-hospital mortality (OR, 0.66; 95%CI, 0.45-1.00; P=.047), while the role of all remaining clinical predictors identified on the primary multivariable analysis was confirmed, including HR> 100 bpm ().
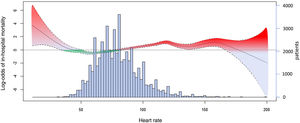
The restricted cubic spline analysis, adjusted for age, sex, creatinine <83μmol/L, OHCA, cardiogenic shock at admission, ACS type, and chronic therapy with beta-blockers or calcium channel blockers, revealed a nonlinear, complex relationship between HR and in-hospital mortality (figure 3). The P-value for the smoothed HR term was <.001.
Restricted cubic spline analysis to model the relationship between heart rate and log-odds of in-hospital mortality, adjusted for age, sex, creatinine <83μmol/L, resuscitated out-of-hospital cardiac arrest, cardiogenic shock at admission, acute coronary syndrome subtype, and chronic therapy with beta-blockers or calcium channel blockers. Bar histogram shows the distribution of the heart rate values.
Three sequential propensity score matched analyses were performed pairing patients with HR <50 bpm and those with HR 50 to 75 bpm, HR 76 to 100 bpm and HR> 100 bpm, identifying 1159, 1159 and 1158 matched pairs, respectively.
report standardized mean differences before and after propensity score matching. After propensity score matching, no differences among rates of in-hospital mortality were evident for groups with admission HR <100 bpm.
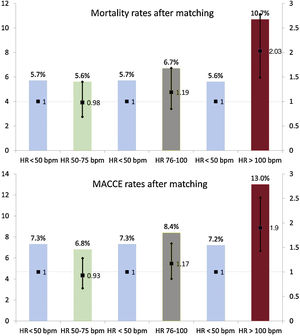
In the matched groups, 66 fatal events (5.7%) were observed among patients with HR <50 bpm, 65 (5.6%) among those with HR 50 to 75 bpm (OR, 0.98; 95%CI, 0.69-1.40; P=.936), 78 (6.7%) among those with HR 76-100 bpm (OR, 1.19; 95%CI, 0.85-1.68; P=.302), and 125 (10.7%) in group HR> 100 bpm (OR, 2.03; 95%CI, 1.49-2.78; P <.001).
Eighty-three (7.3%) MACCE were recorded among matched patients with HR <50 bpm, 78 (6.8%) in the group with HR 50 to 75 bpm (OR, 0.93; 95%CI, 0.67-1.28; P=.683), 96 (8.4%) in group with HR 76 to 100 bpm (OR, 1.17; 95%CI, 0.86-1.58; P=.312), and 147 (13.0%) in those with HR> 100 bpm (OR, 1.90; 95%CI, 1.43-2.52; P <.001). Mortality and MACCE rates along with associated ORs stratified by matched HR group are reported in figure 4. Additional details on outcomes observed in the matched groups are available in .
DISCUSSIONThe availability of a large dataset of patients with MI and a high level of data granularity allowed us to investigate the prognostic role of bradycardia at admission, a parameter which was hypothesized to be associated per se with a deleterious prognostic impact in MI.
In line with reports from several previous registry-based analyses, we observed higher crude adverse event rates in patients with admission HR <50 bpm and> 76 bpm compared with patients with HR 50 to 75 bpm, replicating a bimodal distribution of events, also referred as the “J curve relationship”.7,9–12
However, the restricted cubic spline analysis highlighted a more complex relationship between admission HR and in-hospital mortality. According to our data, while this relationship cannot be oversimplified as a “J-shaped” curve, a certain degree of linearity was observed in patients with HR between 50 to 100 bpm, who account for the vast majority of real-life ACS patients. Interestingly, even when we analyzed HR as a continuous variable, a certain protective effect of HR roughly comprised from 50 to 75 bpm can be claimed, confirmed by negative log-odds ratio estimates for in-hospital mortality.
While the ominous impact of tachycardia (HR> 100 bpm) was strongly confirmed across multiple sensitivity analyses performed among our patients, the signal supporting an independent prognostic role for bradycardia became weaker when we adjusted for multiple clinically relevant covariates. Multiple logistic regression analyses performed after sequential inclusion/exclusion of patients on current medical therapy with drugs with a negative chronotropic effect, such as beta-blockers and calcium channel blockers, did not confirm the independent association between HR <50 bpm and in-hospital mortality, while remaining borderline significant after exclusion of patients with atrial fibrillation.
When we tested analogous groups matched for relevant clinical variables such as age, sex, the presence of renal impairment, ongoing medical therapy, and clinical presentations (OHCA, cardiogenic shock at admission and STEMI), rates of in-hospital events became equal among groups with HR <100 bpm. A progressive fading of any independent prognostic role attributable to HR <50 bpm, along with the “J curve relationship” between admission HR and both primary and secondary endpoints was thus evident after adequate correction of prognostically relevant baseline clinical differences by means of propensity score matching (figure 5).
Central illustration. Overview of a multilevel method with 3 statistical approaches to assess the prognostic role of low HR in ACS including crude, restricted cubic splines and propensity-matched analyses. AMI, acute myocardial infarction; HR, heart rate; MACCE, major cardiovascular and cerebrovascular events.
The direct incremental relationship between HR and adverse events in patients with ACS adopted in the GRACE risk prediction model (which estimates a stepwise increase in the risk of events of 30% for every 30 bpm increase in HR)4–6 was originally challenged by Parodi et al.9 in 2010 while assessing the prognostic impact of HR at admission in 2477 STEMI patients treated with primary percutaneous coronary intervention between 1997 and 2007. After exclusion of patients presenting with AV block and atrial fibrillation, patients with HR <60 bpm (n=388) showed higher in-hospital and 6-month mortality rates compared with patients with HR 60 to 79 bpm. Nonetheless, the relationship between bradycardia and mortality could not be definitely confirmed, as bradycardia was not recognized as an independent predictor of mortality.
Following this initial evidence, Bangalore et al.12 published in 2010 an analysis on 135 164 NSTEMI patients enrolled in the early 2000s in the CRUSADE quality improvement initiative. The limited number of variables collected in CRUSADE did not allow the provision of relevant details regarding the presence of concomitant bradyarrhythmias, atrioventricular blocks, atrial or ventricular tachyarrhythmias or ongoing medical therapy. When stratified according to HR at presentation, significant differences emerged for all baseline characteristics. However, higher adjusted rates of the composite endpoint of all-cause mortality, nonfatal reinfarction and stroke were observed among admission HR <50 bpm compared with those with HR, 60 to 100 bpm (OR, 1.61; 95%CI, 1.23-2.10).
In 2016, a further subanalysis derived from the EURHOBOP project on 10 374 patients with ACS admitted from 2008 to 2010 across 58 European hospitals described a “J-shaped relationship” between admission HR and mortality with the lowest and highest HR conferring the greatest risk.11 This observation was supported by the significant associations observed between HR <40 bpm and crude mortality estimates either in STEMI and NSTEMI patients (STEMI crude OR, 4.3; 95%CI, 1.4-13.2; NSTEMI crude OR, 11.0; 95%CI, 2.3-51.5). Nevertheless, adjusted analyses strongly mitigated this association (STEMI OR, 1.7; 95%CI, 0.5-6.1; NSTEMI OR, 4.7; 95%CI, 1.0-23.6). Also in this case, no details were provided on the presence of associated AV conduction disturbances or other arrhythmias.11
More recently, Perne et al.7 reported the outcomes observed in 6168 patients prospectively enrolled between 2008 and 2014 in the German Chest Pain Unit Registry and stratified according to 4 different HR groups, confirming the “J curve relationship” between admission HR and crude estimates of mortality and MACCE. Again, lack of relevant details regarding concomitant medical therapy at admission did not allow this hypothesis to be confirmed, leading the authors to propose that lower HR might not be causative for the worse outcomes, but rather serves as a marker of increased risk.
All the above-mentioned data are difficult to interpret due to the lack of relevant information on the concomitant presence of atrioventricular conduction disturbances, a factor with a known impact on short-term outcomes.19 In addition, the external validity of the observations was limited by the lack of details regarding history or preadmission use of drugs with a negative chronotropic effect, the low number of patients included in different HR categories, the low event rate in the lowest HR groups, the use of noncontemporary treatment regimens, and the absence of adequate statistical adjustment to balance baseline clinical and ACS related differences among different HR groups.
Thus, our analysis, which reports on a large contemporary collective of patients with ACS analyzed after appropriate statistical correction for baseline differences among different HR groups, strengthened by the results of all sensitivity analyses also evaluating HR as a continuous variable, provides clinically relevant information to solve the “J curve” dilemma.
LimitationsOur findings should be interpreted in the context of the following limitations. First, events were reported by investigators at each site and were not adjudicated by an independent clinical committee. Second, follow-up data were available only in a limited percentage of patients and no data were available to assess the long-term prognostic impact of low HR, a target that was beyond the scope of the present analysis. Third, clinical risk scores recommended by current guidelines, such as the GRACE risk prediction model, could not be estimated using the available data in the registry. Fourth, due to the observational nature of the study, we cannot exclude the presence of confounding factors as an explanation for our results. Due to the registry design, no data were available that would have allowed us to identify and exclude patients with pacemakers apart from those presenting with paced rhythm at admission. However, several adjustments were performed to correct for clinically recognized confounders. Finally, while extensive data regarding ongoing home medical therapy at admission were collected, no data were available on ivabradine, digoxin or amiodarone.
CONCLUSIONSPatients with HR <50 bpm at admission show higher crude in-hospital adverse event rates compared with those with HR between 50 and 100 bpm. Nonetheless, the signal supporting an independent association between bradycardia at admission and short-term mortality is weak and was not confirmed after correction for relevant baseline differences by propensity score matching. The nonlinear, complex association between HR and inhospital mortality in the restricted cubic spline analysis challenges the predictive accuracy of risk prediction scores based on linear models, commonly used in ACS.
FUNDINGThe AMIS Plus registry is funded by unrestricted grants from the Swiss Heart Foundation and from Abbot AG, Amgen AG, AstraZeneca AG, Bayer (Schweiz) AG, Biotronik AG, Boston Scientific AG, B. Braun Medical AG, Daiichi-Sankyo/Lilly AG, Cordis Cardinal Health GmbH, Medtronic AG, Novartis Pharma Schweiz AG, Sanofi-Aventis (Schweiz) AG, SIS Medical AG, Terumo AG, Vascular Medical GmbH, all in Switzerland, and the Swiss Working Group for Interventional Cardiology. The sponsors played no role in the design, data collection, analysis, or interpretation of the data.
AUTHORS’ CONTRIBUTIONSL. Biasco, P. Dittli and G. Pedrazzini designed the study and drafted the manuscript. F. Foster-Witassek and D. Radovanovic acquired and analyzed the data, performed the statistical analysis, and critically reviewed the manuscript. G. Tersalvi, H. Rickli, M. Roffi, F. Eberli, R. Jeger and P. Erne critically reviewed the manuscript. All authors have read and approved the submission of the paper.
CONFLICTS OF INTERESTWe hereby declare that all authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation. None of the authors have any conflicts of interest to disclose.
- •
In patients with ACS, risk prediction scores use incremental models to estimate mortality rates for HR above 60 beats per minute, equalizing the risk for lower HR to that estimated for patients with a HR of 60 bpm.
- •
Nonetheless, numerous studies have described a “J curve relationship” with crude higher event rates at low or high HR.
- •
Thus, if confirmed, the unrecognized prognostic role of bradycardia in MI might potentially hamper the accuracy of clinical and risk score-based predictions.
- •
There is a nonlinear, complex relationship between HR at admission and in-hospital mortality.
- •
The rate of in-hospital events was similar among groups with HR <100 bpm at admission after propensity score matching.
- •
Low HR at admission is not associated with an independent prognostic impact, but rather represents a marker of underlying morbidity.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2023.01.008