The incremental prognostic value of inducible myocardial ischemia over necrosis derived by stress cardiac magnetic resonance in depressed left ventricular function is unknown. We determined the prognostic value of necrosis and ischemia in patients with depressed left ventricular function referred for dipyridamole stress perfusion magnetic resonance.
MethodsIn a multicenter registry using stress magnetic resonance, the presence (≥ 2 segments) of late enhancement and perfusion defects and their association with major events (cardiac death and nonfatal infarction) was determined.
ResultsIn 391 patients, perfusion defect or late enhancement were present in 224 (57%) and 237 (61%). During follow-up (median, 96 weeks), 47 major events (12%) occurred: 25 cardiac deaths and 22 myocardial infarctions. Patients with major events displayed a larger extent of perfusion defects (6 segments vs 3 segments; P <.001) but not late enhancement (5 segments vs 3 segments; P =.1). Major event rate was significantly higher in the presence of perfusion defects (17% vs 5%; P =.0005) but not of late enhancement (14% vs 9%; P =.1). Patients were categorized into 4 groups: absence of perfusion defect and absence of late enhancement (n = 124), presence of late enhancement and absence of perfusion defect (n = 43), presence of perfusion defect and presence of late enhancement (n = 195), absence of late enhancement and presence of perfusion defect (n = 29). Event rate was 5%, 7%, 16%, and 24%, respectively (P for trend = .003). In a multivariate regression model, only perfusion defect (hazard ratio = 2.86; 95% confidence interval, 1.37-5.95]; P = .002) but not late enhancement (hazard ratio = 1.70; 95% confidence interval, 0.90–3.22; P =.105) predicted events.
ConclusionsIn depressed left ventricular function, the presence of inducible ischemia is the strongest predictor of major events.
Keywords
Depressed left ventricular (LV) function is present in about 50% of patients with heart failure1 and the prognosis with ischemic origin is worse than with nonischemic etiology.2 The evaluation for inducible myocardial ischemia appears valuable to discern ischemic from nonischemic etiology of depressed LV function. In normal LV function, prognosis is associated with the presence and amount of ischemia reduction;3 however, the prognostic role of ischemia has not been specifically shown for patients with depressed LV function. Moreover, differentiation between necrotic LV dysfunction vs ischemic LV dysfunction might have important implications since the latter is potentially reversible and thus its reversal may confer symptomatic and prognostic benefit.
Dipyridamole stress perfusion cardiac magnetic resonance imaging (CMR) appears as the ideal noninvasive clinical tool to discriminate ischemic from nonischemic etiology of heart failure. In one examination, CMR allows for a simultaneous assessment of inducible ischemia4,5 and necrosis.6,7 However, apart from these diagnostic considerations, risk stratification in this patient population remains challenging. For that purpose, most studies have focused on myocardial necrosis, but recently it has been demonstrated that the presence of inducible ischemia offers incremental prognostic value.8 However, this issue has not been specifically addressed and a simultaneous assessment of necrosis and ischemia has not been carried out in patients with depressed LV function. Recent studies have pointed out the importance of viability; nevertheless, results were not as decisive as expected and it has been suggested that inducible ischemia might play an important prognostic role.9,10
In a multicenter registry with consecutive patients referred for further diagnostic workup of depressed LV function, we assessed the prognostic value of stress perfusion CMR-derived inducible ischemia and necrosis in terms of major events (cardiac death and nonfatal myocardial infarctions.)
METHODSStudy GroupThis is a multicenter registry conducted in 1 community hospital and 2 university hospitals from January 2003 to June 2010. All baseline data, CMR characteristics, and outcome were collected prospectively according to pre-defined endpoints. Data of patients with depressed LV function, although prospectively collected, were retrospectively reviewed and included: a) consecutive patients with depressed LV function on echocardiography referred for stress perfusion CMR for unclear etiology (ischemic vs nonischemic), and b) patients with depressed LV function and known ischemic heart disease referred for therapeutic decision-making.
Left ventricular ejection fraction was determined on echocardiography using the biplane method of discs (modified Simpson rule) and depressed LV function was defined as < 45%, considered moderately abnormal under current guidelines.11
Patients with acute coronary syndromes, valvular or congenital heart disease, hypertrophic and restrictive cardiomyopathies on echocardiography, as well as acute myocarditis or any contraindications to CMR or dipyridamole, were not included in the study.
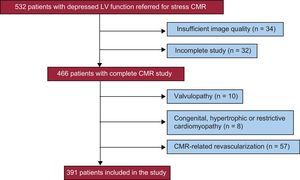
Figure 1 shows the patient flow through the study. Of all included patients, 66 were excluded due to insufficient image quality or an incomplete study. Patients with a final diagnosis of a valvular or congenital heart disease or hypertrophic or restrictive cardiomyopathies previously unrecognized by echocardiography were also excluded.
A CMR-related coronary angiography (prompted by or carried out within 3 months of the CMR examination)4,5 was performed in 122 patients (27%) and was abnormal in 89 cases. To avoid the confounding effect on spontaneous outcome, patients undergoing CMR-related revascularization (37 percutaneous coronary intervention and 20 coronary artery bypass graft) were excluded from analysis. Therefore, the final study population comprised 391 patients.
All baseline characteristics were prospectively recorded upon patient arrival to the CMR facilities by two experienced cardiologists. Management and medical treatment was left at the discretion of the patients’ cardiologists, who were aware of the CMR results. The local ethics committee at each institution approved the study protocol and all subjects gave written informed consent.
Cardiac Magnetic Resonance StudyAll patients were examined with a 1.5 T system (Sonata Magnetom, Siemen, Erlangen, Germany) according to our study protocol.5 Images were acquired by a phased-array body surface coil during breath-holds and were electrocardiogram-triggered.
Baseline cine images were acquired in 2-, 3-, 4-chamber views and in short-axis views using a steady-state, free-precession sequence (repetition time/echo time: 25/1.6ms, flip angle: 61°, matrix: 256 × 256, field of view: 320 × 270mm, slice thickness: 7mm).
Vasodilation was induced with intravenous dipyridamole (0.84mg/kg body weight over 6min). After dipyridamole infusion, 0.1 mmoL/kg gadopentetate dimeglumine (Magnevist®) was injected at a speed of 5mL/s. At least 4 slices in the short-axis view and 2 sections in the 2- and 4-chamber long-axis views were acquired every other beat for stress first-pass perfusion imaging (spoiled gradient-echo, fast low-angle shot (inversion time: 95ms, repetition time/echo time: 172ms/1.34ms, flip angle: 12°, matrix: 192 × 115, slice thickness: 8mm, voxel size 3.0 × 2.1 × 8mm).
Late gadolinium enhancement (LGE) imaging was performed 10min after contrast injection in the same locations used for baseline cine images (segmented, inversion-recovery, steady-state, free-precession sequence (repetition time/echo time: 750/1.26ms, flip angle: 45°, matrix: 256 × 184, field of view: 340 × 235mm, slice thickness: 7mm). Inversion time was adjusted to null normal myocardium.
In order to analyze only reversible perfusion defect (PD) and to exclude fixed PD, resting perfusion was assessed in case of an unclear stress first-pass perfusion study (eg, PD in an area of myocardial necrosis or in the case of extensive subendocardial PD). Acquisition of resting first-pass perfusion was performed at the discretion of the CMR operator at the end of the study using the same sequences as for stress first-pass perfusion.
Cardiac Magnetic Resonance Data AnalysisThe CMR studies were analyzed by 3 experienced observers not blinded to patient data, using customized software (Syngo, Siemen; Erlangen, Germany). The CMR data were prospectively and immediately included into the registry database. Doubtful cases were solved by consensus.
The left ventricular ejection fraction (%), end-diastolic, and end-systolic volume indexes (mL/m2) were calculated by manual planimetry of endocardial and epicardial borders in short-axis views cine images. The CMR indexes were visually defined according to the 17-segment model.12 We determined the number of segments showing PD by persistent delay (in at least 3 consecutive temporal images in comparison with other segments of the same slice) during the first pass of contrast through the myocardium at hyperemia (after dipyridamole-induced vasodilatation).5,13 If resting first-pass perfusion was considered necessary, inducible PD was regarded as a persistent delay of contrast arrival at hyperemia in segments showing normal perfusion at rest.
In LGE imaging, the number of segments showing subendocardial LGE in > 50% of the myocardial wall was determined. This is an accepted cut-off to identify reversible myocardial dysfunction before revascularization.14 Moreover, our group has shown this cut-off to be predictive of a higher rate of events after ST-segment elevation myocardial infarction15 and for systolic recovery in stunned myocardium.16
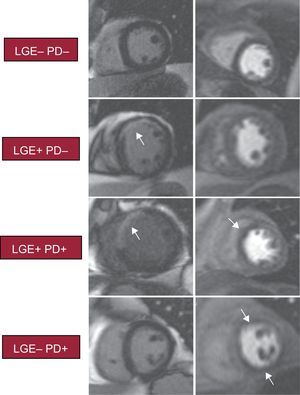
In each segment, LGE was considered present if the signal intensity was >2 standard deviations with respect to a remote noninfarcted area.17 Focal parts of LGE in epicardial areas were not considered in this classification (Figure 2 shows examples of PD and LGE sequences).
Example of cardiac magnetic resonance findings and illustration of the cardiac magnetic resonance categorization. Categorization according to the presence or absence of each index. –, absent (0-1 segment); +, present (≥ 2 segments), LGE, late gadolinium enhancement; PD, perfusion defect.
To avoid misinterpretation of artifacts, significant PD and LGE were only considered to be present, on a per patient basis, if they were detected in both short- and long-axis views and in ≥ 2 segments. In a previous series in our institution,4,6 this was the best cut-off value to predict adverse outcome.
Using cine sequences, the number of segments showing any degree of wall motion abnormality at rest: hypokinesis, akinesis or dyskinesis was determined. In the case of nontransmural necrosis (0 to ≤ 50%), segments showing rest- wall motion abnormalities were considered to be “viable”. For dichotomous analysis, on the basis of a previously validated cut-off value, significant viability in a patient was considered if > 4 viable segments were present.18
The interobserver variability for the determination of the extent (number of segments) of wall motion abnormalities at rest, PD and LGE in our group is < 5%.4,15 The methodology applied for the evaluation of CMR data reproducibility can be consulted in Text 1 of the supplementary material.
End-points and Follow-upFollow-up was centrally performed by 2 cardiologists and 2 trained nurses and updated every 3 months, either from: a) visits in the outpatient clinic; b) a telephone interview with the patient or his/her family, or c) review of the patient's hospital record. To adjudicate an event, consensus between the 2 cardiologists was required.
The primary endpoint of this study was major events, defined as a composite of cardiac death and nonfatal myocardial infarction, whichever occurred first.
Cardiac death was defined as death due to acute myocardial infarction, congestive heart failure, life-threatening arrhythmias or cardiac arrest. Unexpected, otherwise-unexplained sudden death was also considered as cardiac death. Myocardial infarction was defined following current recommendations.19 To avoid the confounding effect on the spontaneous evolution, patients who underwent non-CMR-related revascularization and were free of events at that time were censored.
The main objective of this study was to assess the prognostic value of inducible ischemia (represented by PD) and transmural myocardial necrosis (defined by LGE). Therefore, the following analyses were carried out: First, we analyzed the rate of major events according to the presence (≥ 2 segments) or absence (0-1 segment) of these indexes. Then, in order to assess the combined prognostic value of each index, patients were categorized according to the presence (+) or absence (–) of each index: PD-LGE-, PD- LGE+, PD+ LGE+ and PD+ LGE– (Figure 2).
Statistical AnalysisData were tested for normal distribution using the one-sample Kolmogorov-Smirnov test. Continuous data was expressed as the mean (standard deviation) and compared using the Student t test. Nonparametric data (expressed as median [interquartile range]) was compared with the Mann-Whitney U test or Wilcoxon signed-rank test, as appropriate. Proportions were compared by the chi-square statistic or Fisher exact test, as appropriate. The association of variables with the time to major event was assessed using a Cox proportional hazard regression model with stepwise forward multivariate procedures adjusted for baseline and CMR characteristics yielding a P value < .2 in the univariate analysis. Hazard ratios with the corresponding 95% confidence intervals were computed. Survival distributions for the time to major event were estimated using the Kaplan-Meier method and compared with the log rank test. Statistical significance was considered for P < .05. IBM SPSS (version 20) was used for analysis.
RESULTSOf 391 patients referred for stress perfusion CMR and finally included in the study, 183 patients (47%) were referred for depressed LV function on echocardiography of unclear etiology and 206 patients (53%) had depressed LV function and known ischemic heart disease and were referred for therapeutic decision-making. Of patients with known ischemic heart disease, 123 had undergone previous revascularization (coronary artery bypass graft or percutaneous coronary intervention) and 171 had a history of myocardial infarction. The baseline and CMR characteristics of the study population are shown in Tables 1 and 2.
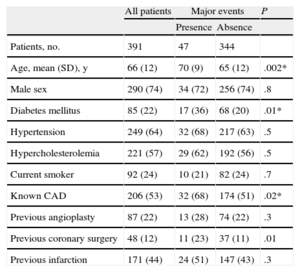
Baseline Characteristics of the Entire Study Population and According to Major Events
| All patients | Major events | P | ||
| Presence | Absence | |||
| Patients, no. | 391 | 47 | 344 | |
| Age, mean (SD), y | 66 (12) | 70 (9) | 65 (12) | .002* |
| Male sex | 290 (74) | 34 (72) | 256 (74) | .8 |
| Diabetes mellitus | 85 (22) | 17 (36) | 68 (20) | .01* |
| Hypertension | 249 (64) | 32 (68) | 217 (63) | .5 |
| Hypercholesterolemia | 221 (57) | 29 (62) | 192 (56) | .5 |
| Current smoker | 92 (24) | 10 (21) | 82 (24) | .7 |
| Known CAD | 206 (53) | 32 (68) | 174 (51) | .02* |
| Previous angioplasty | 87 (22) | 13 (28) | 74 (22) | .3 |
| Previous coronary surgery | 48 (12) | 11 (23) | 37 (11) | .01 |
| Previous infarction | 171 (44) | 24 (51) | 147 (43) | .3 |
CAD, coronary artery disease; SD, standard deviation.
Unless otherwise indicated, data are expressed No. (%) or mean (standard deviation).
P-values are for comparison of patients with and without major events during follow-up using the unpaired Students t test for numeric data and the chi square test for percentages.
*Identifies variables with a P-value ≤ .2 that were used as co-factors in the multivariate Cox regression analysis for predicting time to events. Due to the co-linearity of the variables “known coronary artery disease” and “previous coronary surgery” only “known coronary artery disease” was included.
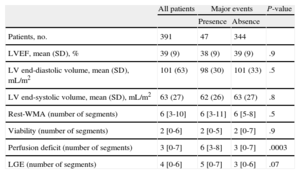
Cardiac Magnetic Resonance Characteristics of the Entire Study Population and According to Major Events
| All patients | Major events | P-value | ||
| Presence | Absence | |||
| Patients, no. | 391 | 47 | 344 | |
| LVEF, mean (SD), % | 39 (9) | 38 (9) | 39 (9) | .9 |
| LV end-diastolic volume, mean (SD), mL/m2 | 101 (63) | 98 (30) | 101 (33) | .5 |
| LV end-systolic volume, mean (SD), mL/m2 | 63 (27) | 62 (26) | 63 (27) | .8 |
| Rest-WMA (number of segments) | 6 [3-10] | 6 [3-11] | 6 [5-8] | .5 |
| Viability (number of segments) | 2 [0-6] | 2 [0-5] | 2 [0-7] | .9 |
| Perfusion deficit (number of segments) | 3 [0-7] | 6 [3-8] | 3 [0-7] | .0003 |
| LGE (number of segments) | 4 [0-6] | 5 [0-7] | 3 [0-6] | .07 |
CMR, cardiac magnetic resonance; LV, left ventricular; LVEF, left ventricular ejection fraction; LGE, late gadolinium enhancement; SD, standard deviation; WMA, wall motion abnormality.
Unless otherwise indicated, data are expressed as mean (standard deviation), or median [interquartile range].
On cine CMR imaging, mean left ventricular ejection fraction was 39% (9%) and LV end-diastolic and end-systolic volumes were 101 mL/m2 (63 mL/m2) and 63 mL/m2 (27 mL/m2), respectively. On stress CMR and LGE imaging, 124 patients (32%) displayed no PD nor LGE. PD (≥ 2 segments) was present in 57% (224 of 391) and LGE (≥ 2 segments) in 61% (237/391), respectively; 195 patients (50%) displayed both PD and LGE. The prevalence of pathologic CMR studies in patients without known CAD is shown in Table 2 of the supplementary material.
Clinical and Cardiac Magnetic Resonance Characteristics of Patients With Major EventsDuring a median follow-up of 96 weeks [37-166 weeks], 47 patients (12%) reached the primary endpoint (25 cardiac deaths and 22 nonfatal myocardial infarctions). Late revascularization (not CMR-related) was performed in 11 patients (6 percutaneous coronary interventions and 5 coronary artery bypass grafts) who did not experience a major event until then and were censored at the time of revascularization.
The baseline and CMR characteristics according to the occurrence of major events are displayed in Tables 1 and 2. Patients with major events were older (70 years [9 years] vs 65 years [12 years]; P = .002),had a higher prevalence of diabetes (36% vs 20%; P = .01) and known coronary artery disease (68% vs 51%; P = .02) with previous coronary surgery (23% vs 11%; P = .01). On CMR, patients with major events displayed a larger extent of PD (6 segments vs 3 segments; P < .001), but only a nonsignificant trend towards a larger extent of LGE (5 vs 3 segments; P = .07).
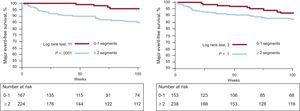
Major Event Rate According to the Presence of Perfusion Defect and Late Gadolinium EnhancementIn the presence of PD (≥ 2 segments) vs its absence (0-1 segments), major event rate was significantly higher (17% vs 5%; P = .0005). In the case of LGE, there was a nonsignificant trend toward a higher rate of major events (14% vs 9%; P = .09).
The Kaplan-Meier analysis showed a significantly longer major event-free survival for patients without PD compared to patients with PD (P < .0001). For LGE, survival did not differ significantly, albeit with a trend toward longer event-free survival in patients without LGE (P = .1, Figure 3).
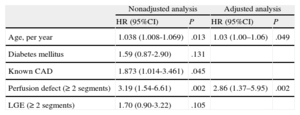
A multivariate Cox proportional hazard regression analysis, adjusted for baseline and CMR parameters yielding P ≤ .2 in the univariate analysis, was performed (Table 3). Of the clinical parameters, age was the only variable independently associated with major events (hazard ratio = 1.03; 95% confidence interval, 1.00-1.06; P = .049, per each life-year increase). The presence of PD was the only CMR parameter independently related to major events (hazard ratio = 2.86; 95% confidence interval,, 1.37–5.95; P = .002); the presence of LGE was not (hazard ratio = 1.70; 95% confidence interval, 0.90–3.22; P = .105).
Univariate and Multivariate Predictors of Major Events*
| Nonadjusted analysis | Adjusted analysis | |||
| HR (95%CI) | P | HR (95%CI) | P | |
| Age, per year | 1.038 (1.008-1.069) | .013 | 1.03 (1.00–1.06) | .049 |
| Diabetes mellitus | 1.59 (0.87-2.90) | .131 | ||
| Known CAD | 1.873 (1.014-3.461) | .045 | ||
| Perfusion defect (≥ 2 segments) | 3.19 (1.54-6.61) | .002 | 2.86 (1.37–5.95) | .002 |
| LGE (≥ 2 segments) | 1.70 (0.90-3.22) | .105 | ||
95%CI, 95% confidence interval; CAD, coronary artery disease; HR, hazard ratio; LGE, late gadolinium enhancement.
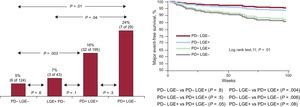
Patients were stratified according to the presence or absence of each PD and LGE: PD- LGE– 124 patients (32%), PD- LGE+ 43 patients (11%), PD+LGE+ in 195 patients (50%) and PD+LGE– 29 patients (7%). The baseline and CMR characteristics of each subgroup are displayed in Table 1 and Table 2 of the supplementary material.
The major event rate across the subgroups showed a steady increase (P for the trend .003) and is depicted in Figure 4. Major event rate in patients with isolated LGE (LGE+PD–) was comparable to patients with no evidence of PD or LGE (PD-LGE-, 7.0% vs 4.8%; P = .6), while it was highest in patients with isolated PD (PD+LGE-). Accordingly, in the Kaplan Meier analysis, adjusted major event-free survival was shorter in the presence of PD (≥ 2 segments) but not of LGE (Figure 4).
Major event rate (left) and Kaplan-Meier analysis (right) for major event free survival according to the combined analysis of perfusion defect and late gadolinium enhancement. –, absent (0-1 segment); +, present (≥ 2 segments); LGE, late gadolinium enhancement; PD, perfusion defect.
This study assesses the prognostic value of inducible ischemia and necrosis as derived from dipyridamole stress perfusion CMR in patients with depressed LV function. The main finding is that inducible ischemia, assessed as PD, was the strongest predictor of major events during follow-up.
Stress Perfusion Cardiac Magnetic Resonance and PrognosisStress perfusion CMR allows for a comprehensive noninvasive assessment of patients with stable coronary artery disease4 and is commonly used for diagnostic purposes.20 Several studies have assessed the prognostic role of inducible ischemia in stress perfusion CMR. In patients with suspected ischemic heart disease, inducible ischemia carries independent prognostic value beyond myocardial necrosis and the absence of ischemia is associated with a low rate of adverse events.7,8,21 However, the role of stress perfusion CMR in depressed LV function is less established. The pathophysiology of heart failure with depressed LV function is multifactorial and in the case of an ischemic etiology involves both necrosis from previous infarction and ischemia from hemodynamic relevant coronary artery disease. In this way, stress perfusion CMR represents a unique diagnostic tool in clarifying the etiology of depressed LV function and may be used for therapeutic guidance22; however, its prognostic value is unclear.
Inducible Myocardial Ischemia in Depressed Left Ventricular FunctionThe therapeutic and prognostic importance of inducible myocardial ischemia has been assessed in several studies. CMR has emerged as the gold standard for the assessment of myocardial necrosis14 and reliably detects PD.5,13 In coronary artery disease, emerging data indicates that benefit from revascularization can only be expected in the presence of significant myocardial ischemia3,23,24 and exploratory data from a large stress perfusion CMR multicenter registry also indicate in that direction.5
In depressed LV function, prognosis is worse in ischemic compared to nonischemic etiology.2 Several modalities have been used to assess myocardial ischemia in patients with depressed LV function25 and the impact of inducible myocardial ischemia in these patients has been indirectly assessed using dobutamine stress echocardiography26 or visualized using single-photon emission computed tomography,27 yielding controversial results. We assessed the prognostic usefulness of stress perfusion CMR-derived inducible ischemia in depressed LV function. Inducible ischemia was associated with a significantly worse prognosis. Though speculative, stress CMR might allow for the identification of a subgroup of patients at exceedingly high risk who might benefit from further work-up, including myocardial revascularization.
Late Gadolinium Enhancement Imaging in Depressed Left Ventricular FunctionCardiac magnetic resonance detects LGE, representing myocardial necrosis with exceptional accuracy, and this technique has been identified as the main determinant of functional recovery after revascularization14 over other techniques defining viability, like end-diastolic wall thickness or low-dose dobutamine.28
In reperfused myocardial infarction, the extent of transmural necrosis has a strong negative prognostic value.15,29,30 In parallel, in severely reduced LV function of ischemic origin, the extent of myocardial necrosis in LGE-CMR was a predictor of adverse events.31 We found a comparable prognosis to a negative LGE and PD study when LGE was present in the absence of any PD. This seems to contradict previous studies, reporting a strong prognostic impact of LGE.31 However, it is important to stress differences in patient selection and methodology. While previous studies used LGE-CMR in highly selected patients with severely depressed LV function of clear ischemic etiology, our results indicate that, in unselected patients with depressed LV function, the mere presence of LGE in the absence of PD is associated with good prognosis while the actual determinant of an elevated risk for future events is the presence of PD.
Clinical ImplicationsIn stable, symptomatic coronary artery disease and preserved LV function, revascularization only improves prognosis in the presence of significant myocardial ischemia. Whether this is true for patients with depressed LV function is unknown. A definition of viability based on myocardial necrosis might not be sufficient in this scenario. Indeed, the STICH trial32 and its viability substudy33 have questioned the utility of viability testing in patients with depressed LV function of ischemic origin. In this study, better outcomes of revascularization were found in the absence of myocardial viability. The fact that the viability protocol did not consistently address inducible ischemia in this trial might have contributed to these findings.
LimitationsA limitation of the study is that patients were only followed for major cardiac events and that baseline characteristics/follow-up did not include a documentation of symptom severity (angina) or the decision-making process the patient or the clinician in charge of the patient made in the knowledge of CMR data. We also did not assess the use of implantable cardioverter defibrillators during follow-up and therefore cannot determine the potential impact on outcome. We aimed to assess the prognostic implications of the presence of a PD and not the prognostic effect of revascularization. Recent randomized trials3 have shown little benefit of imaging-derived ischemia and only demonstrated a prognostic benefit in the case of significant ischemia in a subanaylsis.
CONCLUSIONSIn a consecutive all-comer population of patients with depressed LV function referred for stress perfusion CMR, the presence of inducible myocardial ischemia in a simultaneous assessment of inducible myocardial ischemia and necrosis is the strongest predictor of major events in terms of cardiac death and nonfatal myocardial infarction.
CONFLICTS OF INTERESTNone declared.
Vicent Bodi was supported by the grants PI1102323 (by the Instituto de Salud Carlos III), FEDER and PROMETEO/2013/007 (by the Generalitat Valenciana) and Francisco J. Chorro by the grant RIC (Red de Investigación Cardiovascular) by the Instituto de Salud Carlos III. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.