The idea of including several drugs in a fixed-dose combination for cardiovascular prevention was born at the beginning of this century. Although the suggestion initially received scant attention, the seminal work of Wald and Law in 2003, which introduced the term polypill, attracted the interest of researchers and the media, sparking a controversy that continues today.1 For some health professionals, the idea of a polypill for cardiovascular prevention is merely an interesting concept that is of limited usefulness and applicability, a chimera, which is defined by the official Spanish dictionary of the Real Academia Española de la Lengua as “aquello que se propone a la imaginación como posible o verdadero, no siéndolo” (“something entertained by the imagination as possible or real, but which is not”). For others, however, the polypill could save thousands of lives if used in the proper context and with the correct indication.
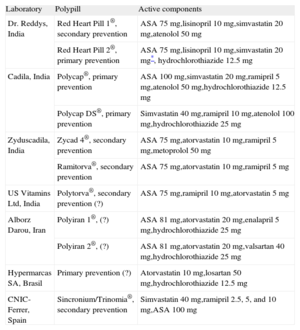
Regardless of the debate, the polypill is now a reality; in fact, various polypills are already available (Table 1). One such polypill has been developed in collaboration between the Spanish National Center for Cardiovascular Research (Centro Nacional de Investigaciones Cardiovasculares [CNIC]) and the pharmaceutical company, Ferrer. This polypill contains acetylsalicylic acid, simvastatin, and ramipril, already forms part of the therapeutic arsenal of various Latin American countries: Mexico, Guatemala, Nicaragua, the Dominican Republic, Honduras, El Salvador, and Argentina. Registration of a second polypill that replaces simvastatin with atorvastatin has been approved in Spain and Sweden, among other European countries, and will be available for prescription during the present year.
Polypills Already Developed
| Laboratory | Polypill | Active components |
| Dr. Reddys, India | Red Heart Pill 1®, secondary prevention | ASA 75mg,lisinopril 10mg,simvastatin 20mg,atenolol 50 mg |
| Red Heart Pill 2®, primary prevention | ASA 75mg,lisinopril 10mg,simvastatin 20mg*, hydrochlorothiazide 12.5mg | |
| Cadila, India | Polycap®, primary prevention | ASA 100mg,simvastatin 20mg,ramipril 5mg,atenolol 50mg,hydrochlorothiazide 12.5 mg |
| Polycap DS®, primary prevention | Simvastatin 40mg,ramipril 10mg,atenolol 100mg,hydrochlorothiazide 25 mg | |
| Zyduscadila, India | Zycad 4®, secondary prevention | ASA 75mg,atorvastatin 10mg,ramipril 5mg,metoprolol 50 mg |
| Ramitorva®, secondary prevention | ASA 75mg,atorvastatin 10mg,ramipril 5 mg | |
| US Vitamins Ltd, India | Polytorva®, secondary prevention (?) | ASA 75mg,ramipril 10mg,atorvastatin 5 mg |
| Alborz Darou, Iran | Polyiran 1®, (?) | ASA 81mg,atorvastatin 20mg,enalapril 5mg,hydrochlorothiazide 25 mg |
| Polyiran 2®, (?) | ASA 81mg,atorvastatin 20mg,valsartan 40mg,hydrochlorothiazide 25 mg | |
| Hypermarcas SA, Brasil | Primary prevention (?) | Atorvastatin 10mg,losartan 50mg,hydrochlorothiazide 12.5 mg |
| CNIC-Ferrer, Spain | Sincronium/Trinomia®, secondary prevention | Simvastatin 40mg,ramipril 2.5, 5, and 10mg,ASA 100 mg |
ASA, acetylsalicylic acid; (?), unspecified indication.
Why, then, does the polypill controversy continue? Reservations about this cardiovascular prevention strategy can be explained by various factors (Table 2), but a decisive part has clearly been played by the different interpretations of the role of the polypill and its possible indications, which have appeared in the literature since the article by Wald and Law.
Arguments For and Against the Polypill Concept
| For |
| Better patient adherence to treatment |
| Reduction in treatment complexity for patients with multiple medications |
| Improved ease of prescription |
| Greater medication availability in developing countries |
| Lower cost |
| Lower medication cost compared with generics in certain countries, particularly developing countries |
| Reduced health care cost due to the reduction in cardiovascular events with improved adherence and prevention |
| Against |
| In the “preventive strategy” |
| Risks of systematic administration to an entire population without previous assessment: |
| • Medicalization of a “healthy” population |
| • Adverse psychological effects |
| • Negative effect on healthy lifestyles |
| • Fear of adverse reactions |
| In primary prevention |
| Absence of studies that prove its efficacy and acceptance by patients and professionals: |
| • Overly optimistic expectations |
| • Cost-effectiveness |
| Difficulty of selecting drugs and doses |
| Difficulty of identifying suitable patients (indications) and the level of risk required for beginning therapy |
| Negative effect on healthy lifestyles |
| In secondary prevention |
| Difficulty of individualizing doses and achieving guideline recommendations |
The initial proposal of Wald and Law involved administration of a polypill with 6 active components to all individuals older than 55 years, regardless of their risk factors and with no need to know their cholesterol levels or blood pressure values.1 The proposal—defined by the authors as a “preventive strategy”—has found strong opposition among health care professionals because of the unknown consequences of medicalizing an entire population, particularly the costs of possible adverse reactions, psychological effects in a healthy population, and the possible promotion of unhealthy lifestyle habits.2 Without suitable clinical studies demonstrating its efficacy, this strategy is unlikely to gain the acceptance of health care professionals and authorities in the near future.
Based on Wald and Law's initial idea, various authors proposed a more selective use of polypills in individuals without cardiovascular disease but with high cardiovascular risk (primary prevention).3 There is no definitive proof of the usefulness, safety, or cost-effectiveness of this approach, although its feasibility has been shown in several pilot studies. However, these studies included patients with and without previous cardiovascular disease, which hampers interpretation of the results; overall, the studies show that polypills increase treatment adherence by 30%, without consistent results on blood pressure or lipid level control. None of these studies had the power to detect differences in the rate of new coronary events. Therefore, the results of studies currently underway are required to determine whether the polypill can play a role in the primary prevention of coronary disease.3–5
Finally, polypill use has been advocated for patients with cardiovascular disease, particularly those who have already had a myocardial infarction (secondary prevention).6 This strategy is gaining supporters for the following reasons:
- •
Patients with coronary heart disease, particularly those with a prior myocardial infarction, should take the 4 (cardioprotective) drugs with demonstrated effectiveness in reducing mortality and preventing new events: acetylsalicylic acid, statins, angiotensin-converting enzyme inhibitors, and beta-blockers.7–9
- •
The PURE (Prospective Urban Rural Epidemiology) study demonstrated that, of more than 5600 patients with previous coronary disease recruited in 17 countries with distinct per capita income levels, more than 60% failed to receive any of these drugs; only 3% received all 4. The lowest income countries showed the worst figures: up to 80% of patients received no drugs of any type after a myocardial infarction.10
- •
The causes of inadequate prevention in those countries are clear. First, health system access is poor and, therefore medical attention is also deficient. For example, in the Study on Prevention on Recurrences of Myocardial Infarction and Stroke (WHO-PREMISE) of the World Health Organization, cholesterol levels were determined in less than 40% of postinfarction patients in certain countries. Moreover, medication is unavailable or too expensive, given that health care coverage in those countries is practically nonexistent and drugs in the private sector are expensive. Consequently, workers may need more than half of their monthly income to buy postinfarction medication.11 Thus, any attempt to apply individualized medicine in those countries according to our standards is a pipe dream. The experience with the CNIC-Ferrer polypill in this type of country shows that price can be reduced by 50% from that of generic drugs in the private sector while improving access, thereby increasing the number of patients receiving better secondary prevention. Even when the costs of the polypill and of each drug separately are similar (in the public sector), the polypill will be more cost-effective due to the reduction in cardiovascular events caused by improved adherence.
- •
Despite the efforts of health care authorities, health care professionals, and scientific bodies, the situation in developed countries is still far from ideal. In these countries, secondary prevention is lacking for 2 main reasons: inadequate prescribing and lack of patient adherence to treatment. Moreover, poor adherence of physicians to clinical guidelines has been revealed in numerous studies, particularly international and national registries, including those supported by the Spanish Society of Cardiology (Sociedad Española de Cardiología) and its Ischemic Heart Disease Section (Sección de Cardiopatía Isquémica).12 Despite improvements in recent years, close to 50% of patients still do not receive beta-blockers or angiotensin-converting enzyme inhibitors following an acute myocardial infarction. Moreover, inadequate prescribing also results in deficient control of risk factors and worse prognosis.
- •
Lack of treatment adherence in patients is a serious problem that has been overlooked in recent decades. The problem is most apparent in patients with chronic diseases and has been detected in all countries studied, irrespective of the health care system, economic situation, and education level. A recently published meta-analysis showed that adherence among patients with cardiovascular disease was 57% (95% confidence interval, 50%-64%) after a mean follow-up of 24 months.13 Similar figures have been found in many other studies, which moreover have analyzed the cause of this nonadherence. Together with the type of drug, one of the main reasons for treatment discontinuation is its complexity and, particularly, the number of doses (ie, capsules, tablets) that the patient must take every day. Claxton et al14 reviewed 76 publications on adherence, concluding that it is inversely proportional to the number of daily doses. Accordingly, a polypill could improve adherence.
- •
Proper treatment adherence is associated with better risk factor control and lower morbidity and mortality in patients with acute myocardial infarction. The beneficial effect of strict adherence to medication has been demonstrated with each of the cardioprotective drugs, both alone and in combination. Thus, the data from the German registry published by Zeymer et al15 are illuminating. Among 9998 patients who were discharged after a myocardial infarction (all treated with beta-blockers), 1-year mortality was 4.9% in patients that received acetylsalicylic acid, an angiotensin-converting enzyme inhibitor, and a statin, 9.7% in those that took 2 of these drugs, and 13.6% in those that took 1 or none.15 Other registries and prospective studies have confirmed this relationship between adherence and prognosis after an acute myocardial infarction.
- •
A fixed-dose drug combination tablet or polypill improves adherence. Various studies of patients with hypertension or diabetes have shown greater treatment adherence with fixed-dose combinations than with separately administered drugs. Moreover, recent new data have confirmed that the polypill effectively improves adherence. The results of the UMPIRE study, involving 2004 patients at high risk of a coronary event, have confirmed that the polypill increased adherence from 65% in the control group to 86% in the polypill-treated group.16 A second more recent study with this same polypill has confirmed the significant improvement in adherence in patients with and without previous cardiovascular disease.17
- •
In contrast to what occurs in primary prevention, the most important regulatory agencies do not require efficacy studies for polypill use in secondary prevention. Only bioequivalence must be shown between the new preparation and drugs administered separately, as their efficacy is well-known and proven. Nonetheless, obtaining a polypill that fulfills these requirements is not a trivial matter and requires extensive experience in pharmaceutical development. In fact, of all of the polypills being developed worldwide, only those of the CNIC-Ferrer project have achieved approval and registration in countries different from their country of origin.
- •
Besides the polypill, other interventions have been proposed to improve treatment adherence in patients with cardiovascular disease. These strategies are based on providing the patient with written information, videos, talks, frequent telephone communication, or personalized advice to motivate correct medication use. Studies generally show that adherence improves with these interventions, but the benefit disappears over time unless continuously performed. These strategies require multidisciplinary teams and a considerable investment of time and resources, limiting their widespread use and clearly making them unthinkable for health care systems in developing countries.
Thus, there appear to be sufficient and valid reasons for incorporating the polypill approach into secondary prevention: improved treatment accessibility and affordability in developing countries and increased treatment adherence, poor in all socioeconomic spheres, which increase event occurrence and health care costs.
The prevention and management guidelines for cardiovascular disease, recently published by the American Heart Association/American College of Cardiology, stress the need to administer high doses of statins (atorvastatin 80mg) to all patients with a prior myocardial infarction, ignoring the strategy of achieving the concrete targets of low-density lipoprotein cholesterol established in previous guidelines.18 This new recommendation is considered a major setback for the use of polypills, which include moderate statin doses (simvastatin 40mg, atorvastatin 20mg). The polypill is not a treatment strategy designed to replace the individualized and personalized prevention recommended by guidelines, only possible in some countries and for specific treatment-adherent patients, but is rather a health intervention that makes prevention available to a large part of the marginalized population worldwide or to Western individuals that show poor adherence, despite having access to medication. Thus, the selection of drugs to be included in a polypill and their doses is based on a compromise between effectiveness and safety, which permits their administration to patients with poor access to medical attention or without sufficient discipline. Although high statin doses (eg, 80mg of atorvastatin) are safe, the rate of certain adverse effects (eg, increase in liver enzymes) is higher than with lower doses.15 Moreover, the relationship between statin dose and the reduction in low-density lipoprotein cholesterol is not linear: low-density lipoprotein cholesterol is decreased by 40% with 20mg of atorvastatin but by 52% with 80mg of atorvastatin .19 This nonlinearity may explain why studies comparing low-to-moderate statin doses with high statin doses based on the recommendations of the American Heart Association/American College of Cardiology fail to demonstrate a reduction in mortality, despite lowering the rate of combined events.
In summary, the polypill is clearly useful for improving treatment accessibility and patient adherence in secondary prevention. In developing countries, the polypill could be a health care intervention with considerable social and economic effects. It may even mark a turning point in the growth of the epidemic and its cost. In the West, the polypill will help to improve adherence in numerous patients with established cardiovascular disease. The reduction in events in these patients will make the polypill a cost-effective option in developed countries. Thus, there should be no conflict between polypills and personalized secondary prevention: both are complementary strategies that can be used by physicians to combat the cardiovascular disease epidemic. The polypill is already a reality and has a great future.
CONFLICTS OF INTERESTSThe authors have no personal conflicts of interests. The CNIC has a project to develop polypills with Ferrer in a public-private partnership.