The most common genetic cause of premature coronary artery disease (CAD) is familial hypercholesterolemia (FH), an autosomal dominant condition in which half the offspring of an affected individual will also be affected from birth. The causative mutations are mainly found in the LDL receptor gene (LDLR) and less frequently in the apolipoprotein B gene and the proprotein subtilisin/kexin type 9 gene (PCSK9). The prevalence of heterozygous FH ranges from 1 in 300 to 1 in 500 individuals in the general population,1,2 and this condition is estimated to affect at least 100 000 people in Spain.3 Furthermore, FH accelerates atherosclerotic coronary disease by 10 to 40 years.2 In Spain, 55% of men and 24% of women with FH between 50 and 59 years of age have had some clinical manifestation of CAD, such as myocardial infarction and chest angina.4 Patients with homozygous FH, which affects approximately 1 per million inhabitants, have total cholesterol levels > 500mg/dL and very premature CAD. Untreated, these individuals die before they are 20 years old. Therefore, FH is a public health problem, and diagnosis and treatment are mandatory.
Diagnosis of FH is based on high concentrations of low-density lipoprotein cholesterol (LDL-C), family history of hypercholesterolemia, presence of premature CAD, and cholesterol deposition in the form of xanthomas and/or arcus senilis.3 Early diagnosis allows preventive measures to be taken. If patients with FH and no history of CAD are treated with statins, the risk of CAD is reduced by 79%, to a level similar to that of the general population.5
Although many recent guidelines for the management of FH have highlighted the high associated cardiovascular risk, most patients with FH remain undiagnosed and untreated.1–3,6 There are a series of barriers to diagnosis and treatment. First, patients with most severe FH are usually first identified in specialist care or lipid clinics, whereas most patients are attended in primary care. Many individuals and family members with FH who have CAD have other common risk factors and so genetic hypercholesterolemia is not diagnosed. In the case of treatment, statin doses are insufficient and combination treatment is used too sparingly.7 Moreover, therapy is often started in the late stages of disease, when atherosclerosis has already developed as a result of life-long high LDL-C concentrations. Finally, health care systems are not sufficiently aware of the problem and there is a lack of screening programs.
From the point of view of public health, the best strategy for covering this gap in diagnosis and treatment of FH is the implementation of a family-based cascade screening program. This process consists of diagnosing FH in the family members of an individual, the index case (IC), identified as having FH. Few countries have implemented national programs for genetic detection through cascade screening. The Netherlands has the best-established program, which began in 1994 and has identified more than half the FH population through genetic screening.8,9 In Spain, a regional genetic screening program was established in 2006, but with varying levels of implementation among the different autonomous regions.
DETECTION OF FAMILIAL HYPERCHOLESTEROLEMIA THROUGH CASCADE SCREENINGThe detection of FH meets the criteria for systematic screening for a disease. The most cost-effective way of identifying new cases of FH is cascade screening of the family members of an IC (proband), using a strategy based on cholesterol concentrations and/or genotyping. An opportunistic search for ICs can be made by in all adults with total cholesterol > 300mg/dL, premature CAD, or tendon xanthomas in those individuals and their family members. In Spain and other countries, use of the criteria of the Dutch Lipid Clinic Network is recommended to identify the IC, with subsequent genetic confirmation.3 This is one of the limiting steps for implementing a cascade screening program, in which awareness of the clinical criteria for diagnosis when HF is suspected is crucial.
Detection based on genetic study can establish definitive diagnosis of FH and assist cascade screening, and is very cost-effective.10 If only LDL-C levels are used for cascade screening, up to 20% of family members with LDL-C levels below the 90th percentile but with a positive LDLr mutation may go undiagnosed.11 Xanthomas are pathognomonic for FH but only present in < 30% of cases with genetic diagnosis of FH.7 There may also be a relationship between genotype and phenotype severity, with a mutation in the null allele usually associated with more severe FH.12
The best strategy for diagnosing family members is therefore a combination of LDL-C levels and genetic analysis when a known mutation is present, provided sufficient resources are available. Drawing up a family tree can be very useful in planning the screening process.
Ideally, FH should be detected before cardiovascular disease develops, but this is rarely the case in everyday clinical practice. Diagnosis in children is therefore important and, when one of the parents has already been diagnosed, screening should start at the age of 2 years and preferably be complete before the child is 8 years old.2,3
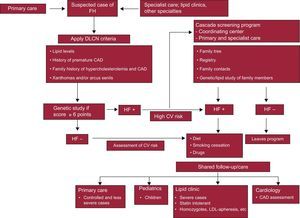
IMPACT AND CONSEQUENCES OF THE DEVELOPMENT OF A CASCADE SCREENING PROGRAMDespite the growing interest in the investigation of FH, care of patients and their family members remains inadequate and substantial improvement in health services at all levels of care is needed. For a cascade screening program to be successful, the primary care system must be involved, engaging the family physician in the identification of the IC and in subsequent diagnoses when screening family members (Figure). Most CAD-free patients with FH can be identified by the family physician. The availability of clinical and laboratory data in electronic format in the health system provides an efficient means of opportunistic searching for undiagnosed patients. Most patients with FH whose management is straightforward can be attended in the long term in primary care. These patients are thought to account for 80% of all patients with FH. To improve the care of these patients, primary care physicians should be trained in the diagnosis and treatment of FH.
Schematic representation of the program for screening and management of familial hypercholesterolemia. CAD, coronary artery disease; CV, cardiovascular; DLCN, Dutch Lipid Clinic Network; FH, familial hypercholesterolemia; LDL-apheresis, low-density lipoprotein cholesterol apheresis.
Specialists in lipid clinics should also participate, but too few such clinics exist in Spain and this must be taken into account. Moreover, they are not evenly distributed throughout the country and no provision is made by the Spanish health system for access to them. A similar situation can be found in neighboring countries. A study in the United Kingdom found that < 15% of patients with FH are treated by lipid specialists.6
In opportunistic screening in a hospital setting, the cardiologist may play an important part in the detection of FH in patients younger than 60 years with CAD and hypercholesterolemia. Moreover, collaboration with a cardiologist interested in FH can improve monitoring and assessment of subclinical coronary atherosclerosis in asymptomatic patients with FH, especially through noninvasive imaging tests, although these require validation in such patients.3
One consequence of cascade screening will be increased detection in children and adolescents. The need for counseling and treatment will require the participation of primary care pediatricians with an interest in metabolic diseases. Primary care pediatricians can treat and monitor through to adulthood those pediatric patients with uncomplicated, well-controlled FH.
Cost-effectiveness studies provide clear support for systematic screening for FH. Thus, genetic diagnosis of family members of patients with identified mutations combined with an analysis of cholesterol is very cost-effective.9 Recently, cost modeling in the United Kingdom estimated that intensive statin treatment of 1000 patients with FH (aged between 30 and 85 years) would prevent 101 cardiovascular deaths compared to the option of no treatment. Overall, this avoidance of coronary events could help save 480 million euros over a 55-year period if all family members of ICs were identified and treated, a saving of almost 9 million euros per year.13
CURRENT SITUATION OF FAMILIAL HYPERCHOLESTEROLEMIA SCREENING IN SPAINSince chronic treatment for FH became available at no cost to patients in 2004, awareness of the condition has increased and diagnosis has received an impetus. Approximately 20 000 individuals are thought to be diagnosed with FH in Spain, accounting for approximately 20% of the estimated population with FH. Of these, more than 60% were diagnosed with clinical criteria.
In recent years, some autonomous regions have implemented different strategies for detection of FH, including genetic diagnosis. This has led to genetic identification of 7000 individuals with FH, the largest number of genetic diagnoses after the Netherlands.9 However, there is no homogeneous program and cascade screening is very limited. Castile and Leon is the only region in which the screening strategy involves primary care physicians. In 2009, this region, in collaboration with the Familial Hypercholesterolemia Foundation, implemented the FH Screening Program, which includes training for physicians and the creation of an ad-hoc registry. The primary care physician or specialist selects ICs according to the criteria of the Dutch Lipid Clinic Network.3 Once a potential IC is identified, genetic study is requested using a saliva sample taken at the health center. If diagnosis is confirmed, cascade screening is triggered. To date, almost 1000 patients have been diagnosed with FH by genetic study in Castile and Leon. Assessment of the results of the program is pending.
Since 2004, the Familial Hypercholesterolemia Foundation (a nonprofit patient organization) has set in motion a national program in collaboration with specialist hospital clinics for cascade screening for FH in the framework of a translational research project known as SAFEHEART Study.7 When the IC is identified using clinical criteria, diagnosis is confirmed through a genetic test and informed consent is obtained to contact family members. The telephone calls are made from the Familial Hypercholesterolemia Foundation and an appointment is arranged with the hospital clinics. To date, 4155 individuals from 771 families have been recruited (mean of > 5 individuals per family).14 Around 3000 individuals in this study have a positive genetic test. Approximately half the family members detected are managed in primary care. An important finding in this study is that, although this is a hereditary disease, approximately 25% of the family members detected were unaware that they had FH and 20% were not receiving lipid-lowering therapy.
This national program of family screening has a data registry and a central repository of biological samples. Demographic, laboratory, treatment, and clinical data are collected at baseline and annual follow-up visits. This novel and innovative study will provide information on cascade screening and so help overcome shortcomings and deficiencies in awareness, detection, and treatment of FH.
The prospective SAFEHEART study will help provide a better definition of prognostic factors of cardiovascular morbidity and mortality,15 and will enable identification of patients who have severe FH in need of more intensive treatment and who stand to benefit from the development of new lipid-lowering drugs.16 In addition to patients with homozygous FH, the study will also define those with severe heterozygous FH and/or progressive atherosclerosis who might be candidates for the option of LDL apheresis.
The information from this program of cascade screening for FH, coordinated by the Familial Hypercholesterolemia Foundation, can help in drawing up policies of prevention and promotion of healthy lifestyles. Along with the program in Castile and Leon, the FH screening program should act as a stimulus for a unified national strategy for FH screening.
RECOMMENDATIONS AND CONCLUSIONSThe lack of diagnosis creates a barrier for the effective prevention of premature CAD and impacts the quality of life and economic and social contributions of individuals and families with FH. Failure to diagnose this condition comes with huge health costs, such as those associated with provision of cardiac and coronary care, coronary revascularization procedures, and management of other vascular episodes.
Greater awareness of the problem among those responsible for healthcare provision is required to improve the diagnosis and treatment of individuals and families with FH. At a time when resources for health interventions need to be prioritized, we should avoid the temptation to opt for high-technology interventions and new treatments at the expense of prevention programs. In the era of genomic medicine, the introduction of new sequencing technologies will significantly reduce the cost of genetic screening.
Familial hypercholesterolemia is a public health challenge that can affect multiple family members and that can be readily diagnosed and treated. Given that most individuals with FH, particularly young people, are asymptomatic, primary care physicians need to get involved and receive training in the appropriate management of families with FH. Most individuals with FH should be treated in primary care, preferably with a focus on the family as a whole. Children and patients with more complex disease should be attended in specialist centers or clinics. For a national screening program, centralized coordination between primary care physicians, specialist care, and nursing staff is needed, along with a patient support organization. An integrated model for the diagnosis and care of FH at a national level, clearly understood by professionals in both primary and specialist care, would have a significant impact on the risk of CAD and healthcare costs and, above all, would prevent premature deaths.
Given the recent history of cascade screening in Spain, and to avoid regional inequalities in the care of family members with FH, we believe that the moment has come to put into practice a national plan for FH screening. Diagnosis and treatment of families with FH is a unique opportunity in the field of preventive medicine.
CONFLICTS OF INTERESTNone declared.