Aortic regurgitation (AR) is a highly-prevalent valve disease in our setting. Surgery is indicated in cases of clinical deterioration or worsening of parameters on 2-dimensional (2D) echocardiography.1 Patients with AR may remain asymptomatic for decades, so early detection of subclinical deterioration may improve outcomes.1 Myocardial deformation parameters on 3-dimensional (3D) imaging represent a promising tool that provides additional information besides left ventricular ejection fraction (LVEF), integrating ventricular geometry from a single apical window, but its usefulness in AR is not yet known.
Between March 2013 and July 2014, we carried out a prospective single-center observational cohort study of consecutive patients with at least moderate AR (≥ III/IV) and LVEF > 55%. The patients were asymptomatic and did not meet the classic criteria for surgery. The study was approved by the ethics committee at our hospital.
We performed 2D echocardiography and 3D assessment of ventricular strain and determined global longitudinal strain (GLS), global circumferential strain (GCS), global radial strain (GRS), and global area strain (GAS) using a Vivid E9 scanner (General Electric Vingmed Ultrasound, Norway) and the software EchoPAC (4DAutoLVQ-EchoPAC BT12, General Electric Vingmed Ultrasound). The same operator performed all studies, meaning that intraobserver and interobserver reproducibility could not be assessed. The primary outcome was the composite of cardiovascular death, hospitalization for heart failure (HF), or ventricular dysfunction during follow-up with LVEF <50% or symptoms attributable to the valve lesion such as deterioration in New York Heart Association (NYHA) functional class, syncope, or angina recorded in the clinical notes.
The study included 31 patients (mean age, 61±18 years; 74.2% were male), 61.3% had a tricuspid aortic valve and 16 (51.6%) had grade IV AR. Up to July 2019, cardiac surgery was indicated in 12 patients (38.7%), all of whom had grade IV AR. The indication was based on clinical deterioration in 7 patients (4 with HF, 3 with NYHA deterioration), worsening ventricular function in 2, and a combination of clinical and echocardiographic factors in 3 (LVEF + NYHA). All hospitalizations for HF met the criteria for surgery. No patients died from cardiovascular causes.
The study was repeated in asymptomatic patients who did not undergo surgery, at 6 months in 17 patients and at 1 year in 10. No significant differences were observed in any of the 3D strain parameters when baseline echocardiogram was compared with those at 6 and 12 months.
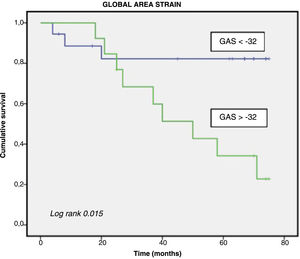
Table 1 shows the comparison according to incidence of the composite outcome. Regarding 3D strain, the only parameter associated with the composite event was GAS. After study of the diagnostic yield using the ROC curve, the optimal cutoff point was a GAS>-32, with 75% sensitivity and 73.7% specificity, area under the curve =0.76 (P=.05), and C statistic=0.64.
Data at baseline and compared according to incidence of composite event
| Variable | Total (n=31) | Event | ||
|---|---|---|---|---|
| No (n=19) | Yes (n=12) | P | ||
| Age, y | 61.61±18.46 | 58.94±17.51 | 65.83±19.89 | .320 |
| Male | 23 (74.2) | 15 (78.94) | 8 (66.66) | .676 |
| Height, cm | 166.52±9.64 | 168.53±7.42 | 163.33±12.05 | .199 |
| Weight, kg | 70.48±13.23 | 72.37±11.66 | 67.50±15.46 | .327 |
| Hypertension | 24 (77.4) | 12 (63.15) | 12 (100) | .026* |
| Diabetes | 1 (3.2) | 1 (5.26) | 0 (0) | 1 |
| Dyslipidemia | 13 (41.9) | 8 (42.10) | 5(41.66) | .981 |
| Lung disease | 4 (12.9) | 3(15.78) | 1 (8.33) | 1 |
| Renal failure | 3 (9.7) | 1 (5.26) | 2 (16.66) | .543 |
| 2D echocardiography | ||||
| Systolic blood pressure, mmHg | 137.97±21.59 | 136.63±23.12 | 140.27±19.51 | .664 |
| Diastolic blood pressure, mmHg | 69.37±13.92 | 69.68±14.14 | 68.82±14.18 | .873 |
| Heart rate, bpm | 72±15.29 | 71.21±14.78 | 73.36±16.79 | .717 |
| LV end-diastolic diameter, mm | 5.54±0.88 | 5.40±0.80 | 5.77±0.99 | .291 |
| LV end-systolic diameter, mm | 3.29±0.75 | 3.24±0.76 | 3.36±0.77 | .663 |
| LV end-diastolic volume, μL | 154.16±46.25 | 144.42±33.38 | 169.57±59.89 | .143 |
| LV end-systolic volume, μL | 44.46±20.74 | 40.97±19.36 | 50.00±22.50 | .245 |
| LVEF Simpson, % | 64.84±6.83 | 66.54±1.62 | 62.14±5.70 | .080 |
| TAPSE, mm | 2.49±0.46 | 2.52±0.48 | 2.43±0.45 | .718 |
| Ascending aorta | 3.4±0.55 | 3.41±0.58 | 3.39±0.53 | .929 |
| Sinotubular junction | 3.05±0.55 | 2.97±0.56 | 3. 18±0.52 | .341 |
| Sinuses of Valsalva | 3.47±0.591 | 3.34±0.13 | 3.70±0.51 | .117 |
| Vena contracta | 0.55±0.13 | 0.56±0.13 | 0.66±0.06 | .011 |
| E/e | 12.05±4.81 | 11.42±4.18 | 14.35±7.22 | .161 |
| AR pressure half-time | 535.08±323.94 | 578.84±381.19 | 465.79±199.69 | .353 |
| Tei index | 0.21±0.89 | 0.20±0.09 | 0.21±0.08 | .821 |
| 3D echocardiography | ||||
| End-diastolic volume, μL | 125.03±50.01 | 121.75±41.99 | 130.24±62.36 | .653 |
| End-systolic volume, μL | 57.9±26.685 | 41.84±19.12 | 54.22±30.83 | .177 |
| EF, % | 62.06±5.97 | 63.49±5.20 | 59.81±6.36 | .095 |
| GLS | –16.96±2.53 | –17.63±2.36 | –15.91±2.53 | .066 |
| GCS | –20.77±4.13 | –21.68±3.59 | –19.33±4.67 | .125 |
| GRS | 54.54±12.06 | 57.89±11.20 | 49.25±11.88 | .050* |
| GAS | –32.64±4.80 | –34.21±4.39 | –30.16±4.52 | .020* |
| Outcome | ||||
| Death | 4 (12.9) | 1 (5.26) | 3 (25) | .272 |
AR, aortic regurgitation; GAS, global area strain; GCS, global circumferential strain; GLS, global longitudinal strain; GRS, global radial strain; LV, left ventricle; LVEF, left ventricular ejection fraction; TAPSE, tricuspid annular plane systolic excursion.
Values are expressed as No. (%) or mean±standard deviation.
The only significant predictors of events in the univariate analysis were vena contracta and GAS>–32. Both were introduced into a multivariate Cox proportional hazards model with backwards stepwise selection with no significant differences found.
The Kaplan-Meier curves showed differences in event-free survival when GAS>–32 (figure 1). Inclusion of a higher number of cases of grade IV AR (at baseline, 51.6%; final, 64.5%) could explain the greater risk of events than in the literature.
In our study, the GAS in AR identifies patients with greater probability of needing surgery, before deterioration in LVEF, which may help improve prognosis. To the best of our knowledge, this study is the first to evaluate a 3D ventricular mechanical parameter, GAS, as an independent predictive factor in patients with asymptomatic AR who do not meet the criteria for surgery.
Two features that could explain why GAS would provide more information than 2D strain studies are that the images are acquired in the same echocardiographic plane evaluating the same cardiac cycle and that acquisition of this parameter is only reproducible with 3D technology.2–4 GAS reflects change in subendocardial area based on information on longitudinal and circumferential shortening measured simultaneously, which provides better predictive power than if measured separately, even with a lower number of patients.
The prognostic value of GAS has been demonstrated in other clinical contexts, although reference values vary according to the different echocardiographic equipment and authors.3,4 In patients with asymptomatic severe mitral regurgitation and preserved EF, a GAS>-41.6% has been found to be predictive of worse outcomes (hazard ratio=4.41; P=.004).5
In conclusion, GAS is the ventricular function parameter that best predicts this composite outcome, even better than the parameter that is usually used, LVEF. Determination of GAS does not vary during follow-up of patients who remain asymptomatic and do not meet criteria for surgery.
We thank Dr Pedro Caravaca Pérez for his thorough review of the manuscript and his suggestions.