Pulmonary hypertension (PH) is often associated with heart failure (HF) and is a known predictor of morbidity and mortality in patients with acute HF (AHF).1,2 Invasive measurement of pulmonary pressures is considered the gold standard for the diagnosis of PH but is not routinely performed in patients with HF, and echocardiography-based estimation is the most commonly used noninvasive method. However, PH study by estimating systolic pulmonary arterial pressure (sPAP) on echocardiography is not always feasible, complicating PH assessment in these patients. When sPAP can be calculated, its values correlate poorly with invasive measurements.3 Consequently, there is increasing interest in the development of other noninvasive imaging indexes to estimate pulmonary arterial pressure. The aim of our study was to evaluate the prognostic value of the indexed pulmonary artery (PA) diameter obtained by cardiac magnetic resonance imaging (cMRI) in patients with AHF.
A total of 1229 patients were admitted to our hospital due to AHF from April 2009 to October 2014. In all, 313 (25%) of these patients were prospectively included if cMRI was performed as part of the etiologic study for HF during hospitalization once they had been stabilized. Bright-blood anatomic sequences were used to measure the indexed PA diameter, and the maximum diameter was calculated for the vessel perpendicular to the longitudinal axis of the common pulmonary artery. Patients were grouped into 4 quartiles according to indexed PA diameter, expressed in mm/m2: Q1 (6.90-12.69), Q2 (12.70-14.59), Q3 (14.60-16.49), and Q4 (16.50-45.40). Echocardiography was also used to calculate left ventricular end-diastolic and end-systolic diameters, left ventricular ejection fraction (LVEF), and sPAP when tricuspid regurgitation was present (n=179). The correlation between sPAP and indexed PA diameter was assessed by the Spearman correlation coefficient. The primary endpoint of the study was to determine all-cause mortality during follow-up. A multivariate analysis was performed with Cox regression models in which all the variables listed in table 1 were evaluated, with the final model including variables with P <.10 and all variables known to be prognostic, regardless of P value. The final multivariate models included the following covariables: age, sex, Charlson index, prior hospitalization for AHF, heart rate, systolic blood pressure, estimated glomerular filtration rate, N-terminal pro-brain natriuretic peptide, and LVEF. The discriminatory capacity and the calibration of the model were adequate (Harrell's C-statistic=0.748; Hosmer-Lemeshow=0.379). The additional reclassification ability of indexed PA diameter compared with standard multivariate models was assessed by the net reclassification improvement.
Baseline characteristics
| Patients, n | 313 |
| Personal history | |
| Age, ya | 70 [60-76] |
| Male sexa | 191 (61.0) |
| Hypertension | 240 (76.8) |
| Diabetes mellitus | 149 (47.6) |
| Dyslipidemia | 188 (60.1) |
| Active smoker | 46 (15.9) |
| Former smoker | 100 (34.6) |
| Chronic ischemic heart disease | 124 (42.9) |
| Prior hospitalization for AHFa | 143 (45.7) |
| Charlson indexa | 2 [0-3] |
| Vital signs | |
| Heart rate, bpma | 82 [70-95] |
| Systolic blood pressure, mmHga | 135 [115-155] |
| Diastolic blood pressure, mmHg | 78 [70-91] |
| ECG and echocardiography | |
| Atrial fibrillation | 89 (28.4) |
| Bundle-branch block | 93 (29.7) |
| LVEF, % | 37 [28-50] |
| LA diameter, mm | 42 [36-47] |
| sPAP, mmHgb | 40 [33-51] |
| cMRI | |
| LVEF, %a | 36 [24-52] |
| LVEDV, mL | 110 [78-140] |
| LVESV, mL | 70 [38-103] |
| RVEF, %c | 50 [39-60] |
| RVEDV, mLc | 65 [49-88] |
| RVESV, mLc | 33 [21-51] |
| Lab work | |
| Hemoglobin, g/dL | 12.6 [11.2-14] |
| Sodium, mEq/L | 139 [136-141] |
| eGFR (MDRD), mL/min/1.73 m2a | 68 [51-88] |
| NT-proBNP, pg/mL | 3500 [1799-5858] |
AHF, acute heart failure; cMRI, cardiac magnetic resonance imaging; ECG, electrocardiogram; eGFR, estimated glomerular filtration rate; LA, left atrium; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end-systolic volume; MDRD, Modification of Diet in Renal Disease; NT–proBNP, N-terminal pro-brain natriuretic peptide; RVEDV, right ventricular end-diastolic volume; RVEF, right ventricular ejection fraction; RVESV, right ventricular end-systolic volume; sPAP, systolic pulmonary arterial pressure.
Data are expressed as n (%) or mean [interquartile range].
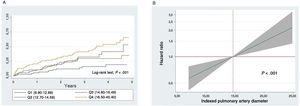
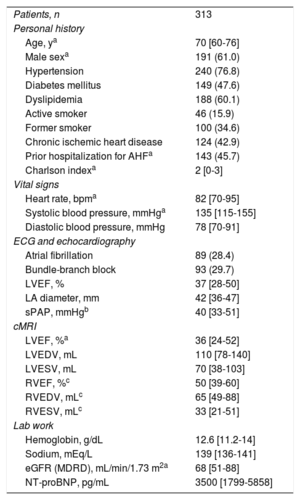
The baseline characteristics of the sample are summarized in table 1. The median age was 70 [60-76] years, 61% were men, and 74.4% had LVEF <50%. During a median follow-up of 2.7 [interquartile range, 1.8-3.7] years, 101 (32.2%) patients died. A stepwise increase was observed in mortality rates (every 10 person-years) from the lower to the upper indexed PA diameter quartiles: 0.49, 1.07, 1.24, and 2.02 for Q1, Q2, Q3, and Q4 respectively (P <.001). This increased risk was observed from the start of follow-up and remained constant (figure 1A). In the multivariate analysis, indexed PA diameter was linearly associated with a higher risk of mortality (hazard ratio=1.07; 95% confidence interval [95%CI], 1.03-1.12; P <.001 per 1-mm/m2increment), as observed in figure 1B. Adding indexed PA diameter to the baseline multivariate models led to significant reclassification (net reclassification improvement=0.324; 95%CI, 0.050-0.610). The correlation between sPAP and indexed PA diameter was positive and weak (r=0.16; P=.048). Two sensitivity analyses included the same covariables plus sPAP, indexed volumes, and right ventricular ejection fraction, showing that indexed PA diameter continued to behave as a parameter independently associated with a higher risk of death.
At present, there is considerable interest in the search for new noninvasive imaging parameters allowing reliable, reproducible PH calculation in patients with HF. Various cMRI-based noninvasive methods have been recently proposed for PH estimation and have shown prognostic value, among them several right-side parameters, although none of them have been clearly confirmed.3 This study is the first in the literature to show a strong positive association between indexed PA diameter and the long-term risk of death in a hospitalized population with AHF. It has been postulated that patients with higher indexed PA diameter values are more likely to experience pulmonary vasculature remodeling and, therefore, combined PH. Our data support measuring indexed PA diameter in all patients with HF who have undergone cMRI, in view of its usefulness for risk stratification. However, this is an observational single-center study, and future research is needed to confirm the usefulness and precision of indexed PA diameter, as well as invasive studies to correlate indexed PA diameter with hemodynamic pulmonary parameters and to define the hemodynamic phenotype most closely corresponding to high indexed PA diameter values. Additionally, there are limitations inherent to cMRI, such as scanner availability or tolerance of the decubitus position by AHF patients.
In conclusion, in patients with AHF, increased indexed PA diameter on cMRI is strongly associated with increased long-term mortality risk.