The role of lung ultrasound (LUS) in acute heart failure (HF) has been widely studied, but little is known about its usefulness in chronic HF. This study assessed the prognostic value of LUS in a cohort of chronic HF stable ambulatory patients.
MethodsWe included consecutive outpatients who attended a scheduled follow-up visit in a HF clinic. LUS was performed in situ. The operators were blinded to clinical data and examined 8 thoracic areas. The sum of B-lines across all lung zones and the quartiles of this addition were used for the analyses. Linear regression and Cox regression analyses were performed. The main clinical outcomes were a composite of all-cause death or hospitalization for HF and mortality from any cause.
ResultsA total of 577 individuals were included (72% men; 69± 12 years). The mean number of B-lines was 5±6. During a mean follow-up of 31±7 months, 157 patients experienced the main clinical outcome and 111 died. Having ≥ 8 B-lines (Q4) doubled the risk of experiencing the composite primary event (P <.001) and increased the risk of death from any cause by 2.6-fold (P <.001). On multivariate analysis, the total sum of B-lines remained independent predictive factor of the composite endpoint (HR, 1.04; 95%CI, 1.02-1.06; P=.002) and of all-cause death (HR, 1.04; 95%CI, 1.02-1.07; P=.001), independently of whether or not N-terminal pro-B-type natriuretic peptide (NT-proBNP) was included in the model (P=.01 and P=.008, respectively), with a 3% to 4% increased risk for each 1-line addition.
ConclusionsLUS identified patients with stable chronic HF at high risk of death or HF hospitalization.
Keywords
Heart failure (HF) is a chronic condition with poor prognosis and frequent hospital admissions.1,2 Patients with established HF often have different stages of clinical pulmonary congestion and even stable patients might have asymptomatic subclinical lung congestion that may go unnoticed.3 The presence of pulmonary congestion may identify those at higher risk of HF hospitalization and death.4 Therefore, diagnosis of any degree of lung congestion is key in the management of patients with chronic HF.
Clinical assessment and complementary tests allow evaluation of patients’ fluid status but have several limitations.5 Decompensation risk scores to rule out congestion are supported by symptoms and physical examination, which are subjective measures and may be absent, especially in ambulatory patients. Natriuretic peptides show significant heterogeneity, even among individuals with similar signs and symptoms, and are not always available in the ambulatory environment. Finally, chest X-ray has high interobserver variability and, moreover, the absence of chest X-ray findings does not exclude the presence of a high pulmonary capillary wedge pressure and congestion.
In this context, lung ultrasound (LUS) appears to be an emergent complementary tool for lung congestion quantification. LUS allows the evaluation of pulmonary congestion by detecting B-lines.6 B-lines are a sonographic artefact caused by the interaction between air and the presence of water in the interstitial space.7 The wide and bilateral presence of B-lines on anterolateral transthoracic lung scans mirror diffuse interstitial syndrome.8 Therefore, LUS may be an alternative for lung fluid retention assessment; it has already been shown to be highly sensitive for pulmonary congestion evaluation in HF patients.9 Moreover, B-lines are associated with changes in both cardiac structure and function,10 and can add prognostic information throughout the spectrum of HF patients.
The role of LUS in the diagnosis and therapeutic approach in the acute HF scenario has been widely studied, especially in the emergency room and critical care wards, where LUS has provided the most evidence to date.4 Nevertheless, there are few data about its value as a prognostic marker in chronic HF outpatients, with most of the data being derived from small cohorts of ambulatory patients.4 Accordingly, we aimed to assess the prognostic value of LUS in a large cohort of chronic HF stable patients. We hypothesized that LUS, performed in situ during routinely scheduled visits in outpatients, might add significant information on prognosis and risk of HF hospitalizations, together with other clinical variables. Moreover, we investigated whether B-lines could provide incremental prognostic information independently of N-terminal pro-B-type natriuretic peptide (NT-proBNP)—which is not always available on-site during outpatient visits—in stable chronic HF patients.
METHODSStudy design and patientsThis is a prospective single center observational cohort study of ambulatory patients attended in a specific HF clinic in a university hospital performed during a routinely scheduled visit from 6 July 2016 to 31 July 2017, independently from the time elapsed between the initial visit at the unit. Referral to the HF unit has been reported elsewhere.11,12 Briefly, the main criterion is having HF with at least 1 hospitalization and/or reduced left ventricular ejection fraction (LVEF) <40%, irrespective of etiology or HF duration. Less than 5% of patients are admitted to the HF unit for asymptomatic reduced left ventricular ejection fraction (LVEF) after acute myocardial infarction. Patients are usually referred to the clinic from the cardiology or internal medicine ward and, less frequently, from the emergency department or other hospital services and also other cardiologists or hospitals of the referral area. All patients are seen regularly during follow-up according to a set schedule and their clinical needs. Structured follow-up includes quarterly based nurse visits and 1 visit from a physician (cardiologist, internist, or family physician) every 6 months, and optional visits from specialists in geriatrics, psychiatry, rehabilitation, nephrology and endocrinology. The annual visit includes an NT-proBNP test, and transthoracic Doppler echocardiogram is performed every 2 years. For clinical needs, patients can spontaneously contact the clinic for an unscheduled visit in there is suspected HF decompensation and can be reassessed as many times as necessary. The clinic has the infrastructure for short-term diuretic and other intravenous drug administration.
For the study purposes, consecutive ambulatory nondecompensated patients who attended a scheduled follow-up visit at the HF clinic were evaluated. Exclusion criteria for the analysis were: a) clinical decompensation at the time of the visit, and b) previous diagnosis of pulmonary fibrosis or radiological diffuse pleural fibrosis.
All participants provided written informed consent, and the protocol was approved by the local ethics committee. The study was performed in accordance with the Guidelines of the Helsinki Declaration of 1975 and its updates in 1983.
Data collectionFollow-up visits were performed by physicians (M. de Antonio, P. Moliner, E. Santiago-Vacas, J. Santesmases) of the HF clinic, who were blinded to LUS result. Clinical evaluation included decompensation assessment, using the HF clinical disease severity score (CDSS)13,14 (decompensated patient: score ≥ 2; each of the major criteria score 1 point [paroxysmal nocturnal dyspnea, pulmonary crackles, elevated jugular venous pressure, third heart sound]; each of the minor criteria score 0.5 points [orthopnea, reduced exercise tolerance, resting sinus tachycardia, jugular venous pressure> 4cm, hepatomegaly, peripheral edema]). Demographic and clinical data were recorded from the electronic medical record review.
Routine annual NT-proBNP tests were analyzed in a central laboratory using an electrochemiluminescence immunoassay (Elecsys, Roche Diagnostics, Switzerland). Biannual LVEF was assess by experienced echocardiographers with a Philips iE33 system using a 3.5MHz transducer, and we included the last obtained before the scheduled follow-up visit.
Lung ultrasound assessmentA single LUS was done in each patient included in the study. LUS was performed in situ during the scheduled visit with a portable pocket device (V-scan simple model with a single sector probe, General Electric, United States) by 1 of the 2 experienced investigators (M. Domingo, L. Conangla), who were blinded to clinical and follow-up visit data. Eight areas established by a previous expert panel8 were examined, and the patients were in semisupine position during the examination. LUS was performed using a phased array transducer, perpendicular to the ribs and an imaging depth of 14cm, and 2-second clip videos were recorded. LUS images were analyzed offline by 1 of the 2 trained investigators (M. Domingo, L. Conangla), who recorded the number of B-lines in the sagittal scan of each thoracic area. B-line was defined as a discrete laser-like vertical hyperechoic reverberation artefact that arises from the pleural line, extends to the bottom of the screen without fading, and moves synchronously with lung sliding.8 The sum of B-lines across all lung zones was used for the main analyses. Pleural effusion was considered as 10 B-lines.
Follow-up and outcomesThe main clinical endpoints were the composite of all-cause death or hospitalization due to HF, as well as mortality from any cause. Follow-up lasted up to 36 months. Mortality events were identified from patients’ electronic records at the HF clinic, other hospital and/or primary care records, and by contacting patients’ relatives. Data were verified using the database of the Catalan health system and the National Death Index (INDEF). Hospital admissions were identified from patients’ electronic records and the database of the Catalan health system. Four of the authors (M. Domingo, M. de Antonio, B. González, and J. Lupón) supervised events adjudication.
Data analysis and statisticsCategorical values are described as absolute numbers (percentages) and continuous variables as means±standard deviation or medians [interquartile ranges], depending on whether the data distribution was normal or nonnormal assessed by normal Q-Q plots. Differences between study groups were analyzed with the chi-square test for categorical variables and with the Student t test or Mann-Whitney U-test for quantitative variables. To assess the relationship between clinical data and the number of B-lines, linear regression or comparison of means (Student t test) were performed, with prior logarithmic transformation of those variables with nonnormal distribution. Univariable and multivariable Cox regression analysis (conditional backward stepwise method) were performed for the defined clinical endpoints. The variable of interest was the sum of B-lines across all lung zones as a continuous variable. On multivariate analysis, 2 models were designed, one with predictive clinical factors in the univariate analysis (P <.1) or considered clinically relevant (age, sex, ischemic HF etiology, New York Heart Association [NYHA] functional class and duration of HF) and another model that also included NT-proBNP as a covariate. The proportionality and linearity assumptions were checked. To meet the linearity assumption, logarithmic functions of NT-proBNP and HF duration were used. Survival curves for all-cause death and for the clinical composite endpoint of all-cause death or HF hospitalization were plotted by dividing the patients in 2 groups according quartiles (Q) of the sum of B-lines, and comparing Q4 (25% of patients with a B-line count> Q3 value) against the remaining patients, taking into account that this cutoff was the best value, based on the area under the curve (AUC). Cumulative incidence curves for the composite clinical outcome of all-cause death or hospitalization due to HF were also represented. Finally, we constructed comprehensive predictive models based on the clinical covariates that remained significant in the Cox regression analyses plus sex, both for the composite endpoint and for all-cause death, and both with and without NT-proBNP entered into the model. Afterwards, we added the LUS total B-line count into the models. Goodness-of-fit was assessed using the Royston modification of Nagelkerke's R2 statistic for proportional hazards models, calibration curves were plotted, discrimination was assessed with Harrell's c-statistic (which takes into account time to the event unlike the usually used AUC). Reclassification was assessed with the continuous Net Reclassification Improvement Index (NRI) and Integrated Discrimination Improvement index (IDI).
Statistical analyses were performed using SPSS 24 (SPSS Inc, Chicago, IL, United States) and STATA V.13.0 (College Station, TX, United States). A 2 -sided P <.05 was considered significant.
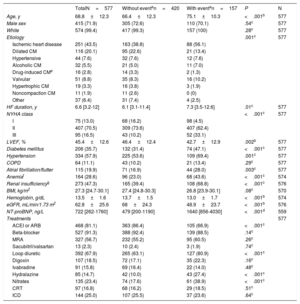
RESULTSDemographics and lung ultrasound association with clinical dataA total of 577 consecutive patients were prospectively evaluated. Table 1 shows the demographic and clinical characteristics of all patients and relative to the composite clinical endpoint of all-cause death or hospitalization for HF. In summary, mean age was 70 years, most patients were male, overweight, and mainly in NYHA class II, with HF predominantly of ischemic etiology. The last LVEF was 45.4%±12.6, although it had been 34%±12.7 at the time of admission to the clinic (baseline visit). The median time since HF onset was 78.8 months (Q1-Q3, 37.8-144.4). Patients were treated according to international guidelines and two thirds received loop diuretics. Patients with an event were older, had more comorbidities and worse renal function, which explains the lower use of angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers and the higher hydralazine administration.
Demographic and clinical characteristics of studied patients
| TotalN=577 | Without eventan=420 | With eventan=157 | P | N | |
|---|---|---|---|---|---|
| Age, y | 68.8±12.3 | 66.4±12.3 | 75.1±10.3 | <.001b | 577 |
| Male sex | 415 (71.9) | 305 (72.6) | 110 (70.1) | .54c | 577 |
| White | 574 (99.4) | 417 (99.3) | 157 (100) | .28c | 577 |
| Etiology | .001c | 577 | |||
| Ischemic heart disease | 251 (43.5) | 163 (38.8) | 88 (56.1) | ||
| Dilated CM | 116 (20.1) | 95 (22.6) | 21 (13.4) | ||
| Hypertensive | 44 (7.6) | 32 (7.6) | 12 (7.6) | ||
| Alcoholic CM | 32 (5.5) | 21 (5.0) | 11 (7.0) | ||
| Drug-induced CMe | 16 (2.8) | 14 (3.3) | 2 (1.3) | ||
| Valvular | 51 (8.8) | 35 (8.3) | 16 (10.2) | ||
| Hypertrophic CM | 19 (3.3) | 16 (3.8) | 3 (1.9) | ||
| Noncompaction CM | 11 (1.9) | 11 (2.6) | 0 (0) | ||
| Other | 37 (6.4) | 31 (7.4) | 4 (2.5) | ||
| HF duration, y | 6.6 [3.2-12] | 6.1 [3.1-11.4] | 7.3 [3.5-12.6] | .01c | 577 |
| NYHA class | <.001c | 577 | |||
| I | 75 (13.0) | 68 (16.2) | 98 (4.5) | ||
| II | 407 (70.5) | 309 (73.6) | 407 (62.4) | ||
| III | 95 (16.5) | 43 (10.2) | 52 (33.1) | ||
| LVEF, % | 45.4±12.6 | 46.4±12.4 | 42.7±12.9 | .002b | 577 |
| Diabetes mellitus | 206 (35.7) | 132 (31.4) | 74 (47.1) | <.001c | 577 |
| Hypertension | 334 (57.9) | 225 (53.6) | 109 (69.4) | .001c | 577 |
| COPD | 64 (11.1) | 43 (10.2) | 21 (13.4) | .29c | 577 |
| Atrial fibrillation/flutter | 115 (19.9) | 71 (16.9) | 44 (28.0) | .003c | 577 |
| Anemiaf | 164 (28.6) | 96 (23.0) | 68 (43.6) | <.001c | 574 |
| Renal insufficiencyg | 273 (47.3) | 165 (39.4) | 108 (68.8) | <.001c | 576 |
| BMI, kg/m2 | 27.3 [24.7-30.1] | 27.4 [24.8-30.3] | 26.8 [23.9-30.1] | .08c | 570 |
| Hemoglobin, g/dL | 13.5±1.6 | 13.7±1.5 | 13.0±1.7 | <.001b | 574 |
| eGFR, mL/min/1.73 m2 | 62.8±25.6 | 68±24.3 | 48.9±23.7 | <.001b | 576 |
| NT-proBNP, ng/L | 722 [262-1760] | 479 [200-1190] | 1640 [856-4030] | <.001d | 559 |
| Treatments | 577 | ||||
| ACEI or ARB | 468 (81.1) | 363 (86.4) | 105 (66.9) | <.001c | |
| Beta-blocker | 527 (91.3) | 388 (92.4) | 139 (88.5) | .14c | |
| MRA | 327 (56.7) | 232 (55.2) | 95 (60.5) | .26c | |
| Sacubitril/valsartan | 13 (2.3) | 10 (2.4) | 3 (1.9) | .74c | |
| Loop diuretic | 392 (67.9) | 265 (63.1) | 127 (80.9) | <.001c | |
| Digoxin | 107 (18.5) | 72 (17.1) | 35 (22.3) | .16c | |
| Ivabradine | 91 (15.8) | 69 (16.4) | 22 (14.0) | .48c | |
| Hydralazine | 85 (14.7) | 42 (10.0) | 43 (27.4) | <.001c | |
| Nitrates | 135 (23.4) | 74 (17.6) | 61 (38.9) | <.001c | |
| CRT | 97 (16.8) | 68 (16.2) | 29 (18.5) | .51c | |
| ICD | 144 (25.0) | 107 (25.5) | 37 (23.6) | .64c |
95%CI, 95% confidence interval; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; CM, cardiomyopathy; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronization therapy; eGFR, estimated glomerular filtration rate (CKD-EPI equation); HF, heart failure; ICD, implantable cardiac defibrillator; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association; NT-proBNP, N-terminal pro-brain natriuretic peptide.
Data are expressed as No. (%), mean±standard deviation or median [interquartile range].
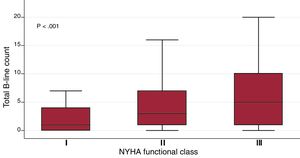
Time between the first visit in the clinic and LUS performance was 69.5±50.8 months (median 59.6 [24.8-98.3]). Only 12 patients had been admitted to hospital in the previous 6 months. The mean number of B-lines in the whole cohort was 5.1±6.1. The distribution of total sum of B-lines per patient is shown in . Quartiles of B-line count were as follows: Q1=1B-lines, Q2=3 B-lines, Q3=7 B-lines. B-lines ranged from 0 (observed in one fourth of the patients) to 31. We found an association between the sum of B-lines with variables such age (β-coefficient 0.11, P <.001), logNT-proBNP (β-coefficient 1.03, P <.001), atrial fibrillation/flutter (mean 6.4±6.5 vs 4.7±6.0, P=.007), loop diuretics (mean 5.7±6.7 vs 4.4±5.8, P <.05), and NYHA class (β-coefficient 1.87, P <.001), and inversely with the log body mass index (logBMI) (β-coefficient –5.0, P=.001). The box plots of the sum of B-lines relative to NYHA functional class are shown in figure 1. The association between B-line sum and logNT-proBNP (R=0.23, P <.001) is shown in . In contrast, we found no association with other variables such as LVEF (β-coefficient 0.004, P=.83), time since onset of HF (log-months β-coefficient 0.14, P=.64) or sex (men 5.0±6.1 vs women 5.3±6.4, P=.64).
Box plot for the sum of B-lines across all lung areas regarding NYHA functional class. The central box represents the values from the bottom to the top quartile; the middle line is the median; T-bars extend to minimum and maximum values, excluding outliers and extremes, which are not shown. NYHA, New York Heart Association.
During a mean follow-up of 31±7.1 months, 111 patients died (26 from HF) and 74 patients experienced at least 1 HF-related admission. The composite endpoint of all-cause death or HF hospitalization occurred in 157 patients.
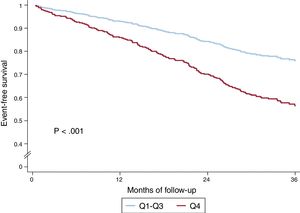
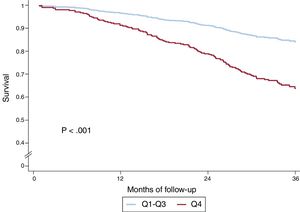
In the univariate analysis, the sum of B-lines showed a significant relationship both with the composite endpoint (HR, 1.05; 95%CI, 1.03-1.08; P <.001) and with all-cause death (HR, 1.06; 95%CI 1.04-1.09; P <.001). shows the results of univariate analyses for both the composite endpoint and for all-cause death. Figure 2 shows the event-free survival curves for the composite endpoint regarding the number of B-lines (Q4 vs Q1-3) and figure 3 the survival curves for all-cause death. Having ≥ 8 B-lines (Q4) doubled the risk of experiencing the composite endpoint (HR, 2.08; 95%CI, 1.50-2.88; P <.001) and increased the risk of death from any cause by 2.6-fold (HR, 2.59; 95%CI, 1.77-3.78; P <.001). In a sensitivity analysis, B-line sum was not related to short-term events (3-6 months), although the number of events was too low to draw robust conclusions.
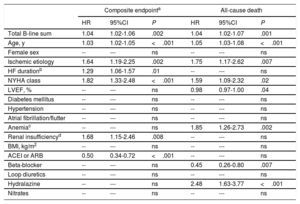
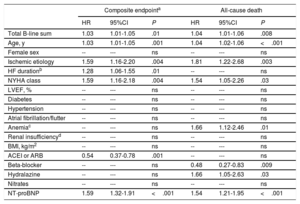
In the multivariate analysis, both including only clinical variables (table 2), and after adding NT-proBNP (table 3), the sum of B-lines was independently associated with the composite endpoint (P=.002 and P=.01, respectively) and with all-cause death (P=.001 and P=.008, respectively), with an increased risk for each 1 B-line addition of 3% to 4%.
Multivariable Cox regression analysis with clinical covariables for the composite endpoint and for all-cause death
| Composite endpointa | All-cause death | |||||
|---|---|---|---|---|---|---|
| HR | 95%CI | P | HR | 95%CI | P | |
| Total B-line sum | 1.04 | 1.02-1.06 | .002 | 1.04 | 1.02-1.07 | .001 |
| Age, y | 1.03 | 1.02-1.05 | <.001 | 1.05 | 1.03-1.08 | <.001 |
| Female sex | -- | --- | ns | -- | --- | ns |
| Ischemic etiology | 1.64 | 1.19-2.25 | .002 | 1.75 | 1.17-2.62 | .007 |
| HF durationb | 1.29 | 1.06-1.57 | .01 | -- | --- | ns |
| NYHA class | 1.82 | 1.33-2.48 | <.001 | 1.59 | 1.09-2.32 | .02 |
| LVEF, % | -- | --- | ns | 0.98 | 0.97-1.00 | .04 |
| Diabetes mellitus | -- | --- | ns | -- | --- | ns |
| Hypertension | -- | --- | ns | -- | --- | ns |
| Atrial fibrillation/flutter | -- | --- | ns | -- | --- | ns |
| Anemiac | -- | --- | ns | 1.85 | 1.26-2.73 | .002 |
| Renal insufficiencyd | 1.68 | 1.15-2.46 | .008 | -- | --- | ns |
| BMI, kg/m2 | -- | --- | ns | -- | --- | ns |
| ACEI or ARB | 0.50 | 0.34-0.72 | <.001 | -- | --- | ns |
| Beta-blocker | -- | --- | ns | 0.45 | 0.26-0.80 | .007 |
| Loop diuretics | -- | --- | ns | -- | --- | ns |
| Hydralazine | -- | --- | ns | 2.48 | 1.63-3.77 | <.001 |
| Nitrates | -- | --- | ns | -- | --- | ns |
ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; HF, heart failure; LVEF, left ventricular ejection fraction; ns, not significant; NYHA, New York Heart Association.
Multivariable Cox regression analysis with clinical covariables + NT-proBNP for the composite endpoint and for all-cause death
| Composite endpointa | All-cause death | |||||
|---|---|---|---|---|---|---|
| HR | 95%CI | P | HR | 95%CI | P | |
| Total B-line sum | 1.03 | 1.01-1.05 | .01 | 1.04 | 1.01-1.06 | .008 |
| Age, y | 1.03 | 1.01-1.05 | .001 | 1.04 | 1.02-1.06 | <.001 |
| Female sex | -- | --- | ns | -- | --- | ns |
| Ischemic etiology | 1.59 | 1.16-2.20 | .004 | 1.81 | 1.22-2.68 | .003 |
| HF durationb | 1.28 | 1.06-1.55 | .01 | -- | --- | ns |
| NYHA class | 1.59 | 1.16-2.18 | .004 | 1.54 | 1.05-2.26 | .03 |
| LVEF, % | -- | --- | ns | -- | --- | ns |
| Diabetes | -- | --- | ns | -- | --- | ns |
| Hypertension | -- | --- | ns | -- | --- | ns |
| Atrial fibrillation/flutter | -- | --- | ns | -- | --- | ns |
| Anemiac | -- | --- | ns | 1.66 | 1.12-2.46 | .01 |
| Renal insufficiencyd | -- | --- | ns | -- | --- | ns |
| BMI, kg/m2 | -- | --- | ns | -- | --- | ns |
| ACEI or ARB | 0.54 | 0.37-0.78 | .001 | -- | --- | ns |
| Beta-blocker | -- | --- | ns | 0.48 | 0.27-0.83 | .009 |
| Hydralazine | -- | --- | ns | 1.66 | 1.05-2.63 | .03 |
| Nitrates | -- | --- | ns | -- | --- | ns |
| NT-proBNP | 1.59 | 1.32-1.91 | <.001 | 1.54 | 1.21-1.95 | <.001 |
95%CI, 95% confidence interval; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; HF, heart failure; HR, hazard ratio; LVEF, left ventricular ejection fraction; ns, not significant; NT-proBNP, N-terminal pro-brain natriuretic peptide; NYHA, New York Heart Association.
summarizes the methodological and LUS key elements of the study, according to expert consensus requirement,15 as well as the main results of the study.
Finally, we constructed comprehensive predictive models as specified in the methods section. Covariables included age, sex, ischemic etiology, NYHA functional class, LVEF, anemia, and treatment with beta-blockers and hydralazine. Both the composite endpoint and all-cause death were assessed with models with and without NT-proBNP. The Royston modification of Nagelkerke's R2 statistic test showed appropriate goodness-of-fit of the models (all P-values between .44 and .60) and the predicted risk closely resembled the observed risk (). Harrell's c-statistic was 0.74 (95%CI; 0.70-0.78) and 0.76 (95%CI; 0.72-0.79) for the composite endpoint, respectively (better if NT-proBNP was added to the model); and 0.77 (95%CI; 0.72-0.82) and 0.78 (95%CI; 0.74-0.83), respectively for all-cause death (again slightly better with NT-proBNP). When we added LUS total B-line count into these predictive models, discrimination was very similar and reclassification was nonsignificant for the composite endpoint (although in the correct direction), but for all-cause death we observed a numerical increase in Harrell's c-statistic in both models with and without NT-proBNP (0.79 (95%CI, 0.74-0.83) and 0.80 (95%CI, 0.75-0.84), respectively). Indeed, reclassification was statistically significant, again for both the model without NT-proBNP (NRI, 0.29 (95%CI, 0.06-0.53), IDI 0.023 (95%CI, 0.001-0.064)) and for the model containing NT-proBNP (NRI, 0.33 [95%CI, 0.002-0.54], IDI 0.019 [95%CI, 0.002-0.059]). These data indicate correct reclassification of ∼10% of patients.
DISCUSSIONFeasibility and general overviewLUS can easily be performed in outpatients and provides in situ information on pulmonary congestion. LUS has been widely evaluated in acute HF, but there are few data on its value in chronic HF. This single center study of LUS in ambulatory stable chronic HF patients shows that it may be a valuable additional prognostic tool in outpatients in conjunction with other clinical variables. Our data suggest that LUS might be more sensitive for the detection of pulmonary congestion than clinical evaluation. Furthermore, B-lines remained as an independent prognostic factor irrespective of NT-proBNP, and showed that LUS might be useful for prognostic stratification in all LVEF spectrum HF outpatients.
Lung ultrasound association with clinical dataAlthough all the patients included in the study were considered stable (CDSS <2), the mean number of B-lines in the cohort was 5.1±6.1, suggesting that LUS might be a better tool for detecting subclinical pulmonary congestion than clinical assessment, similar to previous data.16,17 Moreover, like other studies on acute or chronic HF, we observed that the number of B-lines increased with worsening NYHA functional class and significantly correlated with NT-proBNP levels,18,19 both surrogates for congestion. Our data show that total sum of B-lines was inversely associated with body mass index, as previously reported.6,17,20 Remarkably, a recent publication has shown that the total number of B-lines is less influenced by body mass index than NT-proBNP in acute HF, with a smaller decline than NT-proBNP with increasing body mass index, suggesting that LUS may be useful in patients with HF despite obesity.21 In our study, with 25% obese patients, the correlation between B-line sum and NT-proBNP was statistically significant but rather modest. Another interesting finding of our study is the absence of association with variables such as LVEF, similar to other previous reports,16,17,20 showing that LUS is a valuable tool across the HF spectrum.
Prognostic value of lung ultrasoundIn this study of a cohort of mild-to-moderate systolic HF outpatients (many of them with partially recovered LVEF), we assessed the ability of LUS for detecting patients at high risk of experiencing an adverse event. For this purpose, we carried out a standardized LUS protocol and scanned 8 thoracic areas, analyzing both the anterior and lateral hemithorax. Our results show that the highest quartile of B-lines (≥ 8 B-lines) identified a group of patients with a very high risk of all-cause death or HF hospitalization. Indeed, a higher number of B-lines identified patients with a 2.6-fold increased risk of death from any cause. The sum of B-lines across all lung areas in LUS, together with other clinically relevant prognostic variables, remained as an independent prognostic factor, even when natriuretic peptides were included in the model. These data are relevant, since NT-proBNP is not readily available in many outpatient clinics. Other smaller studies with a shorter follow-up period and patients with a different severity of risk have used a different protocol scan (28-region B-line scan), obtaining similar results regarding the prognostic value of LUS in chronic HF.16,17,22,23 Direct comparison of these studies is not straightforward mainly due to the different LUS study protocol used.
Indeed, discrimination and reclassification tools showed an incremental prediction gain for the estimation of risk of all-cause death, adding LUS to comprehensive clinical predictive models, even in the presence of NT-proBNP. This was not observed for the composite endpoint of all-cause death or HF hospitalization by study design. LUS analyses were blinded to clinical data and therapeutic management was blinded to LUS data. Nevertheless, we cannot exclude the possibility that the researchers may have been aware of the results of LUS. Any modification of management, especially diuretic management, would have an impact on HF hospitalization. Although this possibility is speculative, it may be plausible in day-to-day HF patient management. Indeed, recent data have shown that tailored LUS-guided diuretic treatment in ambulatory patients after a HF hospitalization improves outcomes,24 especially those relative to congestion management.
Based in our findings, we believe that LUS could be integrated in usual clinical practice in HF patients, since is an easy and feasible tool to be used in ambulatory HF consultations. Indeed, previous data suggest that even nonphysicians are usually able to perform and interpret LUS scans after brief training.25
Study limitationsThis was a single centre study with patients who were treated at a specific multidisciplinary HF clinic in a tertiary care hospital, with patients managed with a common protocol. Most of them were referred after at least 1 hospital admission or with a history of difficult management, but many of them also had partially recovered LVEF. We cannot disregard selection bias by disease severity and management and our data cannot be extrapolated to the overall HF population. The number of B-lines may be impacted by clip duration.26 In our study, due to the characteristics of the device, we recorded 2-second clip videos that may underestimate the number of B-lines.
CONCLUSIONSOur data show that LUS, performed in situ during a scheduled visit in stable HF outpatients, is a valuable test and adds significant information on prognosis, together with other clinical variables. Subclinical lung congestion on LUS is usual even in clinically stable patients. B-line measurement with LUS is an independent prognosticator of all-cause death and the clinical composite endpoint of all-cause death or HF hospitalization above and beyond NT-proBNP.
FUNDINGThis work was supported by a competitive grant of La Marató de TV3 (PI 201510.10).
CONFLICTS OF INTERESTNone declared.
- -
LUS is a user-friendly tool that can be easily performed in HF patients and provides in situ information on pulmonary congestion.
- -
LUS has been widely evaluated in acute heart failure, but there are few data on its value in stable chronic HF.
- -
LUS emerged as a better tool for detecting subclinical pulmonary congestion than clinical assessment.
- -
The total number of B-lines is an independent factor of death and the composite endpoint of all-cause death or HF hospitalization.
- -
LUS identifies patients with stable chronic HF at high risk of all-cause death or HF hospitalization across the LVEF spectrum.
- -
Integrating LUS with clinical data in ambulatory patients with chronic HF improves prognostic stratification irrespective of natriuretic peptide availability.
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.rec.2020.07.006.