Amyloid light-chain (AL) amyloidosis is the most common form of cardiac amyloidosis; it is caused by hematological disorders. Cardiac involvement is observed in over 70% of patients at diagnosis and is a marker of poor prognosis.1 Current treatment options are chemotherapy with bortezomib, hematopoietic stem cell transplant, and heart transplant.2
Two-dimensional strain echocardiography is the diagnostic3 and prognostic technique of choice,4 and right ventricle (RV) parameters in particular provide important information.5,6
We investigated the prognostic value of echocardiographic parameters in patients with AL cardiac amyloidosis treated with bortezomib following the detection of cardiac involvement. Prognostic markers would enable the prompt institution of alternative treatments in patients expected to respond poorly to bortezomib and also avoid the use of futile treatment.
AL cardiac amyloidosis was diagnosed by endomyocardial biopsy using standard stains, including immunohistochemical staining with specific kappa and lambda light-chain antibodies. None of the patients underwent spectrophotometry. The primary outcome was death or heart transplant.
We prospectively included 47 patients with AL cardiac amyloidosis, all bortezomib-naïve at the time of echocardiography. Eight patients were excluded because they died before receiving the first cycle of bortezomib. Fifteen patients (38.5%) experienced a primary event (11 [73.3%] died and 4 [26.7%] underwent a heart transplant) during a median follow-up of 697 (interquartile range, 183-1233) days. There were no losses to follow-up. The patients’ characteristics are summarized in table 1.
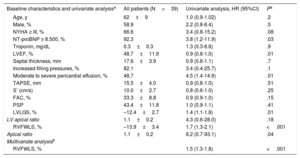
Patient characteristics, univariate analysis of primary events (death or heart transplant) in patients with amyloid light-chain cardiac amyloidosis, and multivariate analysis
| Baseline characteristics and univariate analysisa | All patients (N=39) | Univariate analysis, HR (95%CI) | Pa |
|---|---|---|---|
| Age, y | 62±9 | 1.0 (0.9-1.02) | .2 |
| Male, % | 58.9 | 2.2 (0.8-6.4) | .5 |
| NYHA ≥ III, % | 66.6 | 3.4 (0.8-15.2) | .08 |
| NT-proBNP ≥ 8.500, % | 92.3 | 3.8 (1.2-11.9) | .03 |
| Troponin, mg/dL | 0.3±0.3 | 1.3 (0.3-6.8) | .9 |
| LVEF, % | 48.7±11.9 | 0.9 (0.8-1.0) | .01 |
| Septal thickness, mm | 17.6±3.9 | 0.9 (0.8-1.1) | .7 |
| Increased filling pressures, % | 82.1 | 3.4 (0.4-25.7) | .1 |
| Moderate to severe pericardial effusion, % | 48.7 | 4.5 (1.4-14.9) | .01 |
| TAPSE, mm | 15.5±4.0 | 0.9 (0.8-1.0) | .51 |
| S’ (cm/s) | 10.0±2.7 | 0.8 (0.6-1.0) | .25 |
| FAC, % | 33.3±8.8 | 0.9 (0.9-1.0) | .15 |
| PSP | 43.4±11.8 | 1.0 (0.9-1.1) | .41 |
| LVLGS, % | –12.4±2.7 | 1.4 (1.1-1.8) | .01 |
| LV apical ratio | 1.1±0.2 | 4.3 (0.6-28.0) | .18 |
| RVFWLS, % | –13.9±3.4 | 1.7 (1.3-2.1) | <.001 |
| Apical ratio | 1.1±0.2 | 8.2 (0.7-93.1) | .04 |
| Multivariate analysisb | |||
| RVFWLS, % | 1.5 (1.3-1.8) | <.001 | |
95%CI, 95% confidence interval; FAC; fractional area change; HR, hazard ratio; LV, left ventricular; LVEF, left ventricular ejection fraction; LVLGS, left ventricular longitudinal global strain; NT-proBNP, N-terminal pro-brain natriuretic peptid; NYHA, New York Heart Association; PSP, pulmonary systolic pressure; RV, right ventricular; RVLS, right ventricular longitudinal strain; TAPSE, tricuspid annular plane systolic excursion.
Values are expressed as HR (95%CI) unless otherwise indicated.
All the RV systolic function parameters were reduced. Tricuspid annular plane systolic excursion (TAPSE) showed slightly decreased values, but the differences were not significant. RV longitudinal strain (LS) was reduced in all patients and 59.5% had RV free-wall LS (RVFWLS) of less than 13%. The results of the univariate analysis are shown in table 1.
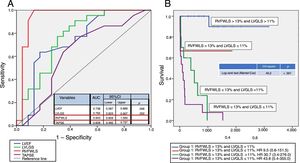
As shown by the receiver operator characteristic (ROC) curves in figure 1A, the parameters with the best sensitivity and specificity for predicting the primary outcomes were left ventricular global longitudinal strain (LVGLS), left ventricular ejection fraction (LVEF), and, in particular, RVFWLS, which had an area under the curve of 0.94 (95% confidence interval, 0.86-1.00).
A, ROC curves showing AUC for LVEF, TAPSE, LVLGS, and RVFWLS. B, Kaplan-Meier survival curves for the combination of LVLGS and RVFWLS using the ROC curve cutoff points. AUC, area under the curve; LVEF, left ventricular ejection fraction; LVLGS, left ventricular longitudinal global strain; ROC, receiver operating characteristic; RVFWLS, right ventricular free-wall longitudinal strain; TAPSE, tricuspid annular plane systolic excursion.
The patients were divided into 4 groups according to the ROC cutoff values for RVFWLS and LVGLS:
- •
Group 1: RVFWLS >13% and LVGLS >11%
- •
Group 2: RVFWLS >13% and LVGLS ≤11%
- •
Group 3: RVFWLS ≤13% and LVGLS >11%
- •
Group 4: RVFWLS ≤13% and LVGLS ≤11%
The survival curves for each of the groups are shown in figure 1B. Patients in group 1 (RVFWLS >13% and LVGLS >11%) had a 5-year survival rate of more than 90%. By contrast, all the patients in groups 3 and 4 (the 2 groups with RVFWLS ≤ 13%) died within 5 years of being diagnosed with AL cardiac amyloidosis.
Hazard ratios (HRs) were calculated to estimate the effect of strain alterations on each of the ventricles (RVFWLS and LVGLS) for group 1 (figure 1B).
Based on the results of the univariate analysis, RVFWLS appears to be the 2-dimensional strain parameter that provides the greatest prognostic information in patients with AL cardiac amyloidosis, although concomitant LVGLS alteration provided a more accurate stratification of the groups with the best prognosis.
Statistically significant and clinically relevant variables with the highest HRs were entered into a Cox regression model using forward stepwise selection. As shown in table 1, the only covariate positively associated with the occurrence of a primary event in the model featuring N-terminal pro-brain natriuretic peptide, moderate to severe pericardial effusion, RVFWLS, LVEF, and LVGLS was RVFWLS, with an HR of 1.51 (95% confidence interval, 1.29-1.76).
In conclusion, RVFWLS is the best prognostic marker for patients with AL cardiac amyloidosis who are candidates for bortezomib chemotherapy. Concomitant LVGLS alteration increases the risk of an unfavorable outcome. Patients with RVFWLS of less than 13% and LVGLS of less than 11% do not respond well to bortezomib and may benefit from an early heart transplant if they show good hematologic response.