The use of cardiac implantable electronic devices (CIEDs) has expanded in recent years. Infection related to these devices constitutes one of the main complications and is associated with high morbidity, mortality, and financial cost. The aim of this study was to construct a predictive risk score of acquiring CIED infection.
MethodsWe designed a retrospective, nested case-control study. Both cases and controls belonged to a cohort that included all patients who underwent a CIED-related procedure between January 2009 and December 2015. Cases were defined as patients with infection, and 3 infection-free controls were randomly selected from the cohort for each case included.
ResultsDuring the study period, 2323 procedures were performed. A total of 33 CIED-related infections were identified. Ninety-nine patients were selected as controls. Independent risk factors were the Charlson index (OR, 1.33; 95%CI, 1.07-1.67), oral anticoagulation (OR, 3.51; 95%CI, 1.44-8.54), revision or replacement of a previous device (OR, 2.75; 95%CI, 1.12-6.71) and the presence of more than 2 leads (OR, 3.42; 95%CI, 1.25-9.37). A predictive risk score was generated and denominated CIED-AI (Charlson Index, more than 2 leads/Electrodes, Device revision/replacement, oral Anticoagulation, previous Infection). This score had an area under the receiver operating characteristic curve of 0.79 (95%CI, 0.71-0.88).
ConclusionsThe CIED-AI score may help to identify patients at higher risk of infection, who could be candidates for intensive preventive measures.
Keywords
The use of cardiac implantable electronic devices (CIEDs) has expanded in recent years due to the increase in the number of patients with conditions that require such interventions.1,2 These include permanent pacemakers (PPMs), implantable cardioverter-defibrillators (ICDs), and cardiac resynchronization therapy (CRT) devices. Infection related to these devices constitutes one of their main complications and causes high morbidity, mortality, and financial cost.3
Despite the systematic observance of strict asepsis in operating rooms, as well as the use of antibiotic prophylaxis, the incidence of these theoretically preventable infections has increased in recent years.4,5 Recently, more complex, expensive and not definitively accepted strategies have been described in an attempt to further reduce these infections.5 These comprise postoperative antibiotics administered through the local and systemic routes and wrapping the device in an antibacterial envelope with rifampicin and minocycline.5 Therefore, identifying patients at higher risk of infection is important when considering the application of these additional measures or even ruling out device implantation in patients with debatable indications.
Several risk factors for CIED infection have been identified over the last few years.6 Some are related to patient characteristics, such as male sex, diabetes mellitus, renal failure, and oral anticoagulation, whereas others are device- or procedure-related (a non-de novo procedure, more than 2 lead device, and ICD or CRT compared with PPM).7–12 However, the risk of infection in each individual may have a greater relationship with a combination of certain risk factors than with the sum total of the risk factors presented in each case.
To our knowledge, 2 studies have been published that describe a composite score to stratify the risk of infection.13,14 In the first, PPM patients were not included because they are supposedly less susceptible to infection than patients with ICD or CRT.13 In the second, the difference between high-risk and low-risk patients did not reach statistical significance.14 Consequently, we contemplated formulating a risk factor based score that, for all types of device, could predict each individual's probability of acquiring infection.
METHODSA retrospective nested case-control study was performed. Both cases and controls belonged to a defined cohort that included all the patients in whom a CIED-related procedure was carried out in a university hospital between January 2009 and December 2015. The procedures studied included de novo implantation as well as CIED revisions or replacements that included PPM, ICD, or CRT.
A retrospective review was performed of all the electronic medical histories related to the previously defined cohort up to December 2016. A case was defined as a CIED-related infection if any procedure related to the CIED (including de novo implantation, revision, or replacement) had been performed within the study period.
CIED-related infection was defined according to modified Duke criteria for CIED-related infection. Local CIED infections (pocket infection) as well as systemic infections (lead-related endocarditis) were included. All cases had a minimum of 2 sets of blood cultures. In all cases, Gram stain and culture of the generator pocket tissue and the CIED lead had been performed when the device was removed. Transesophageal echocardiography was performed in cases of fever or suspected CIED endocarditis to evaluate for lead or valvular vegetations and to inspect the left-sided heart valves even when the transthoracic echocardiogram was normal. All cases had a follow-up of at least 1 year after diagnosis. Early infections were considered to be those occurring before the first year after implantation and late as those occurring after the first year.
The antibiotic prophylaxis protocol in our center consisted of local irrigation of 2 g cefazolin plus a single 400 mg dose of teicoplanin intravenously and 80mg intravenous gentamicin within 60minutes of the surgical incision. The management of the anticoagulation prior the procedure was done according to medical criteria, with no protocol established in our center regarding bridge therapy with heparin.
A control was a defined as a patient in whom a CIED-related procedure was carried out during the cohort period (until December 2015) and who had not developed any CIED-related infection by the time the electronic medical records were reviewed (December 2016). Controls were selected from the initial cohort that included all procedures. Three unmatched controls were randomly selected for each case included in the study. Online random number generator software was used for this selection. The selection of the control were unmatched in order to allow analysis of possible risk factors of infection, such as age or sex, which could not have been analyzed otherwise.
The following demographic and clinical presentation information was collected: age, sex, oral anticoagulation, previous diseases (including history of previous endocarditis or CIED infection, prosthetic cardiac valve), active smoking, obesity (body mass index> 30), previous treatments and recent hospital admissions (defined as a hospital admission within 90 days prior to the procedure) or recent infection (defined as any infection within 90 days prior to the procedure). We measured the Charlson comorbidity index at the time of the procedure.15 Information was also collected with respect to the device and procedure, such as the type of device, number of leads, indication for the procedure, antibiotic prophylaxis, procedure location, type of intervention (de novo device vs revision/replacement), year of intervention, and postprocedure hematoma (we considered postprocedure hematoma when it was described in the electronic medical record by the attending practitioner). The following infection-related information was also included for case patients: clinical presentation, microbiology, treatment, and outcomes. All patients gave consent for their medical histories to be used for research purposes. The study was approved by the local clinical research ethics committee.
Statistical AnalysisQualitative variables are expressed as absolute value and percentage. Quantitative variables are expressed as median and interquartile range (IQR). In the univariable analysis, the qualitative variables were compared using the chi-square or the Fisher exact test when necessary, and odds ratios (ORs) with their 95% confidence intervals (95%CIs) were also obtained. The quantitative variables were compared using the Student t-test or Mann-Whitney U test when necessary. With respect to the multivariable analysis, 2 unconditional logistic regression models were built to include the largest number of clinically important variables associated with the occurrence of infection: the first model included relevant variables related to the patient (eg, demographic variables, previous diseases) and the second included the relevant variables related to the device or the procedure. ORs and 95%CIs were provided for both models.
Based on the results obtained in the multivariable analysis, as well as clinically important variables, we developed a predictive score for the risk of pacemaker infection. The score calibration was assessed using the Hosmer-Lemeshow goodness-of-fit test as well as the discrimination through the area under the receiver operating characteristic curve. Sensitivity, specificity and likelihood ratios were provided.16
P values below .05 was considered statistically significant. All statistical analyses were performed using SPSS version 23 software (SPSS Inc, IBM, Chicago, Illinois, United States).
RESULTSDuring the study period, 2323 procedures were performed in 2193 patients, of which 1591 (68.5%) were carried out in the electrophysiology laboratory and 732 (31.5%) in the operating room. There were 1675 de novo implant procedures (72.1%), of which 1071 (46.1%) corresponded to PPM, 378 (16.3%) to ICD, and 226 (9.7%) to CRT. The remaining 648 (27.9%) procedures were revisions or replacements, of which 339 (14.5%) corresponded to PPM, 230 (10%) to ICD, and 79 (3.4%) to CRT. All patients received antibiotic prophylaxis according to the protocol established in our center.
During the cohort follow-up period, 33 cases of CIED-related infections were detected in 31 patients, representing an infection rate of 1.4%. Two patients had 2 episodes of CIED-related infection during the study period. The infection rate of PPM and ICD were similar (0.9% and 1.0% respectively), but the infection rate of CRT was significantly higher (4.5%, P=.004). Median age was 63.0 years (IQR, 54.0-75.5), 73% were male, with a Charlson index of 4.7 (IQR, 3.4-6.0). The median time between device procedure and device infections was 8 months (IQR, 3-28). The infection was detected during the first year in 20 cases (60.6%), and during the first month in 5 cases (15.2%). Pocket infection was detected in 11 cases (33.3%), endocarditis in 16 (48.5%), and mixed infection in 6 (18.2%). A total of 17 cases (51.5%) had fever, 17 (51.5%) developed local inflammation, and 9 (27.3%) showed renal deterioration (defined as an increase of at least 0.5 mg/dL in baseline creatinine). There was no statistical difference in the frequency of CIED procedures carried out in the electrophysiology laboratory and the operating room (P=.30). There was no statistical difference in the time to device infection between de novo implants and device revisions (median 9.5 months for primary implantation vs 8 months for device revision, P=.957). A total of 6 patients (20%) died during the first year, 3 (10%) of them during hospital admission: 2 due to infection and 1 from cardiogenic shock. The other 3 patients died after hospital discharge: 1 from sudden death, 1 from a noncardiogenic or infectious cause and, in the case of the third, the cause of death was not registered in their electronic record. No patient was lost to follow-up.
Cultures were positive in 30 cases (91%). Blood cultures were positive in 12 cases (36%) (1 from a pocket infection and 11 from lead-related endocarditis). A total of 8 wound cultures and 18 generator cultures were positive, while microbiological isolates were obtained from 19 lead cultures (66%).
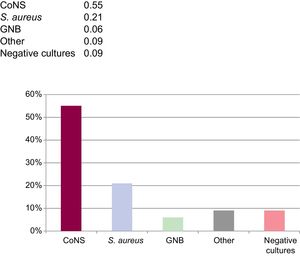
More than 1 bacterial species were identified in 9 cases. Coagulase-negative staphylococci (CoNS) or Staphylococcus aureus were isolated in 25 cases (76.8%). Of these, there were 14 cases of methicillin-resistance, 1 of S. aureus and the rest were CoNS, corresponding to 76% of the CoNS isolated. Two cases of gram-negative bacilli infection (Pseudomonas aeruginosa and Serratia marscenses) were detected, 1 case of streptococci infection, 1 case of infection due to an anaerobic bacteria, and 1 case of fungi infection (Candida albicans). Additional microbiology information is presented in the Figure 1.
A total of 99 unmatched, uninfected controls were selected. The results of the univariable analysis comparing these groups are summarized in Table 1 and Table 2. Limited statistical and clinically relevant variables were selected for the multivariable analyses. The results of the multivariable analyses are summarized in Table 3 and Table 4. Due to the small number of available cases, some variables had to be excluded from this analysis, such as a history of endocarditis, previous CIED-related infection or recent hospital admission. However, we conducted a post hoc multivariable analysis including recent admission, resulting in a P value=.102 with OR 2.20 (95%CI, 0.84-6.10).
Univariate Analysis of Patient-related Variables
| Cases (n=33) | Controls (n=99) | P | Odds ratio(95%CI) | |
|---|---|---|---|---|
| Male sex | 24 (72) | 69 (71) | .829 | 0.89 (0.37-2.15) |
| Age, y | 63.0 [54.0-75.5] | 72.5 [63.7-81.0] | .084 | |
| Charlson index | 4.7 [3.4-6.0] | 4.0 [1.0-5.0] | .004 | |
| Active smoking | 6 (20) | 9 (10) | .200 | 2.25 (0.73-6.95) |
| Alcohol | 6 (18) | 6 (7) | .098 | 3.00 (0.89-10.10) |
| Hypertension | 25 (76) | 66 (68) | .511 | 1.47 (0.60-3.62) |
| Diabetes mellitus | 10 (30) | 34 (35) | .675 | 0.80 (0.34-1.89) |
| COPD | 6 (18) | 14 (17) | .598 | 1.37 (0.46-3.77) |
| Heart failure | 22 (67) | 44 (45) | .044 | 2.41 (1.05-5.51) |
| Ischemic heart disease | 8 (24) | 27 (28) | .821 | 0.83 (0.33-2.06) |
| Atrial fibrillation | 13 (39) | 30 (31) | .394 | 1.47 (0.65-3.35) |
| Prosthetic valve | 6 (18) | 4 (4) | .016 | 5.22 (1.37-19.90) |
| Chronic renal failure | 9 (27) | 21 (21) | .632 | 1.37 (0.56-3.40) |
| Liver failure | 3 (9) | 2 (2) | .101 | 4.80 (0.76-30.10) |
| Obesity | 9 (47) | 36 (56) | .602 | 0.70 (0.25-1.96) |
| Immunosuppression | 2 (6) | 3 (3) | .599 | 2.04 (0.33-12.79) |
| Active cancer | 2 (6) | 11 (11) | .515 | 0.51 (0.11-2.43) |
| Previous CIED infection | 5 (15) | 3 (3) | .01 | 7.38 (1.73-31.54) |
| Previous IE or CIED infection | 6 (19) | 3 (3) | .003 | 11.1 (2.11-58.10) |
| Recent admission* | 13 (39) | 15 (15) | .006 | 3.59 (1.48-8.75) |
| Corticoid treatment | 1 (3) | 3 (3) | 1.000 | 0.99 (0.09-9.85) |
| Oral anticoagulation | 20 (61) | 25 (25) | <.001 | 4.49 (1.95-10.30) |
| Antiplatelet therapy | 13 (39) | 42 (42) | .839 | 0.87 (0.39-1.94) |
95%CI, 95% confidence interval; IE, infective endocarditis; CIED, cardiac implantable electronic device; COPD, chronic obstructive pulmonary disease; y, years.
Qualitative variables are expressed as total number (percentage) and quantitative variables as median [interquartile range].
Univariate Analysis of Procedure- and Device-related Variables
| Cases(n=33) | Controls(n=99) | P | Odds ratio(95%CI) | |
|---|---|---|---|---|
| Urgent indication | 8 (24) | 32 (32) | .393 | 0.66 (0.29-1.63) |
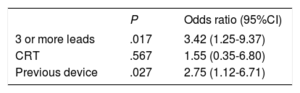
| Device revision/replacement | 19 (57) | 25 (25) | .001 | 3.96 (1.73-9.05) |
| Generator replacement | 17 (51) | 24 (24) | .004 | 3.49 (1.52-8.04) |
| Lead replacement | 6 (18) | 6 (6) | .041 | 3.54 (1.05-11.90) |
| Device upgrade | 2 (6) | 0 (0) | .056 | |
| Lead abandonment | 4 (12) | 5 (5) | .228 | 2.57 (0.65-10.20) |
| Previous temporal device | 4 (12) | 6 (6) | .260 | 2.19 (0.58-8.31) |
| CRT | 14 (43) | 16 (16) | .004 | 3.73 (1.55-8.94) |
| ICD | 6 (18) | 18 (18) | 1.000 | 0.97 (0.35-2.71) |
| PPM | 13 (39) | 65 (66) | .014 | 0.76 (0.60-0.95) |
| 2 or more leads | 25 (76) | 61 (62) | .205 | 1.64 (0.80-3.32) |
| 3 or more leads | 13 (39) | 11 (11) | .001 | 5.14 (2.01-13.20) |
| Left ventricular lead | 14 (43) | 19 (19) | .011 | 3.06 (1.30-7.19) |
| Operating room procedure | 15 (45) | 34 (34) | .302 | 1.57 (0.70-3.50) |
| Postprocedural hematoma | 9 (27) | 13 (13) | .103 | 2.45 (0.94-6.42) |
| Hospital admission, d | 2 [1-10] | 2 [1-6] | .158 | |
| Procedures during admission | 1 [1-1] | 1 [1-1] | .563 | |
| Year of procedure | .95 | |||
| 2009 | 3 (9) | 11 (11.1) | ||
| 2010 | 5 (15) | 15 (15.2) | ||
| 2011 | 4 (12) | 14 (14.1) | ||
| 2012 | 5 (15) | 16 (16.2) | ||
| 2013 | 7 (22) | 14 (14.1) | ||
| 2014 | 4 (12) | 12 (12.1) | ||
| 2015 | 5 (15) | 17 (17.2) |
95%CI, 95% confidence Interval; CRT, cardiac resynchronization therapy; ICD, implantable cardiac defibrillator; PPM, permanent pacemaker.
Qualitative variables are expressed as total number (percentage) and quantitative variables as median [interquartile range].
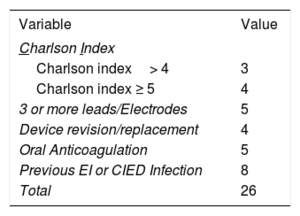
A risk score was developed from these results by taking into account the beta coefficient of the variables found in the multivariable analysis to be associated with the risk of developing a device-related infection. Because it was considered clinically significant, a previous history of endocarditis or cardiac device infection was added to the score.6
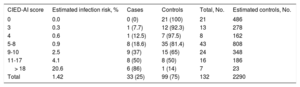
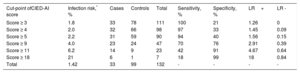
The name for the score was derived from an acronym of the variables included: CIED-AI score (Charlson Index, more than 2 leads/Electrodes, Device revision/replacement, oral Anticoagulation, previous Infection), corresponding to the acronym of Cardiac Implantable Electronic Device-Associated Infection. The CIED-AI score, shown in Table 5, demonstrated good calibration in the Hosmer-Lemeshow goodness-of-fit test (P=.60), and good discrimination with an area under the receiver operating characteristic curve of 0.79 (95%CI, 0.71-0.88). When the score was applied to predict the risk of early CIED infection only, it had a similar area under the receiver operating characteristic curve (0.76; 95%CI, 0.65-0.87). The risk of infection according to each score level was estimated in our population. The likelihood ratios and infection risk were also calculated according to the score cutoff points. These calculations are shown in Table 6 and Table 7, respectively.
Estimated Infection Risk in Our Population According to the CIED-AI Score Value
| CIED-AI score | Estimated infection risk, % | Cases | Controls | Total, No. | Estimated controls, No. |
|---|---|---|---|---|---|
| 0 | 0.0 | 0 (0) | 21 (100) | 21 | 486 |
| 3 | 0.3 | 1 (7.7) | 12 (92.3) | 13 | 278 |
| 4 | 0.6 | 1 (12.5) | 7 (97.5) | 8 | 162 |
| 5-8 | 0.9 | 8 (18.6) | 35 (81.4) | 43 | 808 |
| 9-10 | 2.5 | 9 (37) | 15 (65) | 24 | 348 |
| 11-17 | 4.1 | 8 (50) | 8 (50) | 16 | 186 |
| > 18 | 20.6 | 6 (86) | 1 (14) | 7 | 23 |
| Total | 1.42 | 33 (25) | 99 (75) | 132 | 2290 |
Unless otherwise indicated, data are expressed as No. (%).
Different Possible Cut-off Points of the CIED-AI Score and Their Likelihood Ratio
| Cut-point ofCIED-AI score | Infection risk,* % | Cases | Controls | Total | Sensitivity, % | Specificity, % | LR+ | LR - |
|---|---|---|---|---|---|---|---|---|
| Score ≥ 3 | 1.8 | 33 | 78 | 111 | 100 | 21 | 1.26 | 0 |
| Score ≥ 4 | 2.0 | 32 | 66 | 98 | 97 | 33 | 1.45 | 0.09 |
| Score ≥ 5 | 2.2 | 31 | 59 | 90 | 94 | 40 | 1.56 | 0.15 |
| Score ≥ 9 | 4.0 | 23 | 24 | 47 | 70 | 76 | 2.91 | 0.39 |
| Score ≥ 11 | 6.2 | 14 | 9 | 23 | 42 | 91 | 4.67 | 0.64 |
| Score ≥ 18 | 21 | 6 | 1 | 7 | 18 | 99 | 18 | 0.84 |
| Total | 1.42 | 33 | 99 | 132 | - | - | - | - |
LR+, positive likelihood ratio; LR–, negative likelihood ratio.
The infection risk for each cutoff point can be calculated according to the following formulas (13):
Postscore odds=(LR+)x(population infection risk / 1–population infection risk).
Infection risk=postscore odds / (1+odds postscore).
In these formulas, the infection risk is expressed as points (1.4%=0.014).
Similarly, the infection risk for a score value below the cutoff point can be calculated as:
Postscore odds=(LR–)x(population infection risk / 100–population infection risk).
Infection risk=postscore odds /(1+odds postscore).
In these formulas, the infection risk is expressed as probability, not percentage (for example, 1.4%=0.014).
As an example, in our population a patient with a score value of ≥ 5 would have an infection risk of 2.2%, whereas a patient with a score value <5 would have an infection risk of 0.2%.
This study represents an attempt to determine the probability of CIED infection in individual patients, which would allow exceptional preventive measures to be taken. The possible results of the score allow determination of the probability of a CIED infection that ranges between 0% and 20% (Table 6 and Table 7).
The overall infection rate was low (1.4%), similar to other studies,1,6,17 which may be related to the widespread application of measures to prevent infection.5 Our infection rate of PPM was similar to that found in the Spanish pacemaker registry,1 although we could not compare the ICD or CRT data since these data are not available in the Spanish registry of implantable automatic defibrillators.2 As already established in the literature, there was a clear predominance of CoNS and S. aureus as causes of infection.10,13,17,18 In relation to the type of infection, more cases presented with CIED endocarditis (48.5%) than pocket infection (33%), or mixed infections (18.5%), which does not correlate with previous reports, which describe a higher number of local infections than endocarditis.11,17,19 This could be related to a high proportion of previous hospital admissions (39%), but we cannot definitively rule out the possibility that some cases with mild, localized CIED pocket inflammation were not included in the study.
To the best of our knowledge, this is the first study to identify risk factors of CIED infection in our country. The main risk factors for CIED infection in our series were comorbidity, oral anticoagulation, previous infection, device revision/replacement, and devices with more than 2 leads. Previous studies have identified all these as relevant risk factors.6,8–10 In particular, the implantation of devices with more than 2 leads has been related to longer, more complex procedures.8,10,20 On the other hand, certain recognized infection risk factors, such as advanced age, male sex, diabetes mellitus, heart failure, hypertension, renal failure, or corticosteroid use, were not encountered in our study.6,9,10,13 However, it is possible that the small number of cases and potential differences among the study populations could justify this finding. Other risk factors, such as fever within 24hours of device implantation or early pocket re-exploration, could not be analyzed due to study design limitations.13 We performed the analysis taking into account all episodes of infections because a history of previous CIED-related infection is considered to be an important risk factor,6-10 and excluding the 2 repeated episodes could result in a bias when this factor is analyzed In fact, when performing these analysis excluding the second CIED-related infection on the repeated patients, we obtained equivalent results, except for the previous infective endocarditis or CIED infection variable, which was no longer statistically significant.
Recently presented data indicate that an antibacterial envelope may reduce the infection rate by 80% compared with historical control data.13,21 This positive effect has even been observed using propensity matching for risk of CIED infection13,14 and appears to be economically rational in high-risk patients.22 Results from a large multicenter prospective randomized controlled trial are expected later in 2018 (the WRAP-IT [Worldwide Randomized Antibiotic EnveloPe Infection PrevenTion Trial]), but this trial included only patients with CIED revision/replacement or de novo CRT implants and may thus have excluded other high-risk groups.23 It has been proposed that a score system could be helpful in identifying high-risk patients who may be candidates for the envelope technique.24
Another possible strategy includes postoperative antibiotic prophylaxis. The PADIT (Prevention of Arrhythmia Device Infection Trial) compared conventional antibiotic prophylaxis (cefazolin or vancomycin) with the same regimen plus bacitracin pocket wash and 2 days of postoperative oral cefalexin and is close to publishing its results.25 An additional time-honored preventive action is to screen patients for nasal S. aureus colonization using conventional culture or a polymerase chain reaction test. If patients are found to be colonized with methicillin-sensitive and methicillin-resistant S. aureus, they could undergo decolonization with both nasal mupirocin ointment and chlorhexidine bath for 5 days prior to surgery. If methicillin-resistant S. aureus is identified, vancomycin and cefazolin could be given as prophylaxis.5
All the measures mentioned above could be implemented in cases with a high baseline risk of infection. Even the decision to replace a CIED should be taken considering a risk and benefit approach, counterbalancing the mortality due to device failure, the rate of CIED failure, and the risk of procedure-related mortality. In certain cases, maximizing battery longevity by setting the lower rate limit of heart frequency or other actions may exempt the patient from CIED replacement.5,7,24
To our knowledge, only 2 other scores have been described in the literature. The score described by Mittal et al., 13 included a variable that is unknown at the moment of implantation (ie, early pocket re-exploration) and was built without including PPM patients. Another score system has been used by Shariff et al.,14 who considered high-risk patients to be those who have a score value of≥3, but the difference between groups did not reach statistical significance. Moreover, the score was not developed through a statistical analysis of previous risk factors, but by considering variables associated with a higher risk of infection in a number of previous studies.
In contrast, the score described in our study can easily be calculated preoperatively and has been develop through statistical analysis and, in addition, demonstrates good discrimination between groups. In conclusion, it is easy to apply and, as indicated, it may contribute to better patient management.24 As an example, in a hypothetical population similar to ours, with a total infection risk of 1.4%, a patient with a score value ≥ 5 (positive likelihood ratio, 1.56; negative likelihood ratio, 0.15) would have an infection risk of 2.0%, whereas if the patient had a score value <5, the infection risk would be 0.2%. With a score value ≥ 9 (positive likelihood ratio, 2.91; negative likelihood ratio, 0.39), the patient would have an infection risk of 4.0%, whereas if the score value was <9, they would have an infection risk of 0.5%. The score could also be applied in populations different to ours. As an example, in a hypothetical population with a similar prevalence of risk factors, but with an infection risk of 3%, a score value of ≥ 9 predicts an infection risk of 8.2%, whereas a value <9 predicts a risk of 1.1%. In the same population, a score value ≥ 5 predicts an infection risk of 4.6%, whereas a value of <5 predicts a risk of 0.4%. These calculations can be based on the formulas described in Table 7, as well as by using online calculators.16 We are aware that some cases in our sample had late infections, and the risk factors analyzed in these cases may have less influence. However, most of our cases had early infections, so we believe this score is still useful. Moreover, when the score was applied taking into account only those cases with early infections, it continued to predict in a similar way the risk of infection (data are not shown).
This retrospective study was carried out in a single center thereby narrowing its generalizability, which is an important limitation. Another limitation is the small number of cases included, which could prevent the detection of more clinical differences between cases and controls. Another shortcoming is that some variables were not included, such as operator experience, procedure duration, management of anticoagulation, fever within 24 hours of the procedure or early revision of the surgical site, because these data could not be obtained in a retrospective review of the patients’ electronic records. Finally, the effectiveness of the score has not been validated with an external cohort. However, our investigation group is currently working to strengthen the score's applicability with such an external validation study.
CONCLUSIONIn summary, comorbidity, oral anticoagulation, device revision/replacement, and devices with more than 2 leads were identified as independent risk factors for CIED-related infection. In addition, a previous endocarditis or device infection increased the probability of developing a CIED infection. Those factors may help to identify patients with a greater baseline risk of infection and who could be candidates for extraordinary preventive measures. The CIED-AI score could be highly useful in determining these risks. Additional studies are required to validate the use of this tool.
–CIED-related infections are one of the main causes of morbidity and mortality in patients with these devices, and there is currently strong interest in improving their prevention. Revision or replacement of a device, CRT, an antecedent of device infection, and several comorbidities have been identified in other media as risk factors for these infections.
WHAT DOES THIS STUDY ADD?–In our study, oral anticoagulation, a higher Charlson index, revision or replacement procedures, the presence of more than 2 leads, and a history of infectious endocarditis or CIED infection were identified as risk factors. The proposed CIED-AI score could be useful in the identification of patients at high risk of developing CIED-related infections and who may be candidates for exceptional preventive measures.
J. Toquero Ramos reports personal fees from Medtronic, from Boston Scientific and from Abbott also unrelated outside the submitted study. I. Fernández Lozano reports grants from the Sociedad Española de Cardiología and from the Fundación de Investigación Cardiovascular. He also reports grants and personal fees from Microport, from Biotronik, from Medtronic and from Abbott, outside the submitted study. The remaining of authors nothing to declare.