Pulmonary atresia with a ventricular septal defect is a complex congenital condition associated with a poor prognosis in patients with hypoplastic or nonexistent pulmonary branches and large aortopulmonary collateral vessels. In this situation, a staged strategy is preferred to provide pulmonary flow as promptly as possible and achieve growth and development of the pulmonary arteries before corrective surgery is undertaken.1 The most commonly used traditional palliative surgery techniques were a central systemic-to-pulmonary shunt1 and placement of a transannular patch or a synthetic conduit between the right ventricle and the pulmonary artery. Later, a percutaneous approach was described, consisting of pulmonary valve perforation by radiofrequency2 and stent implantation in the right ventricular outflow tract, as an alternative to surgery. A hybrid approach is used in low-weight newborns, such as the patient described here.
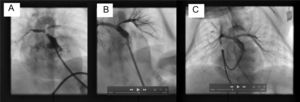
A term neonate weighing 2.6kg was diagnosed with a double outlet right ventricle and an unrelated ventricular septal defect, overriding tricuspid valve, and pulmonary atresia, with compensatory circulation through multiple aortopulmonary collateral vessels. The pulmonary trunk and both branches were hypoplastic (trunk, 2.5mm; branches, 1.3mm). A central shunt and partial correction were ruled out, and a decision was taken to perform a hybrid procedure consisting of a surgical sternotomy and perforation of the pulmonary valve, with periventricular stent placement. Periventricular puncture of the right ventricle was carried out using a 22-G needle. A 0.018-inch guidewire was inserted, enabling passage of a 5-Fr Cook radial introducer, which was placed just below the pulmonary valve (Figure A and ). Contrast material was injected and minimal anterograde pulmonary flow was observed. Passage of a 0.014-inch Whisper coronary guidewire (Abbot) was achieved and the introducer was then advanced through the dilator. Patency of the pulmonary trunk and branches was verified. A premounted 6 × 16-mm Formula stent (Cook) was then implanted in the pulmonary valve. The procedure was successful, achieving adequate perfusion of the pulmonary tree and showing hypoplasia of the left pulmonary branch (Figure B and ). The patient was transferred to the intensive care unit, but extubation was not possible because of excessive pulmonary blood flow. A new catheterization was carried out 12 days after the first, and growth of both pulmonary branches was observed (Figure C and ). The collaterals were successfully embolized with Interlock coils (Boston), thereby allowing extubation and hospital discharge at 20 days following the second procedure. At 6 months of follow-up, the patient was asymptomatic, with arterial oxygen saturation at 82%, and awaiting a partial cavopulmonary shunt.
A, Right ventriculogram with cranial angulation in the posteroanterior view shows minimal anterograde pulmonary flow and the confluence of the trunk and the hypoplastic pulmonary branches. B, Pulmonary arteriogram with cranial angulation in the posteroanterior view depicts correct stent placement and adequate perfusion of both pulmonary branches, although with hypoplasia of the left pulmonary branch. C, The posteroanterior pulmonary arteriography image with cranial angulation at the second catheterization shows that both pulmonary branches have grown and there is adequate perfusion of the lung segments.
Treatment of pulmonary atresia with a ventricular septal defect, hypoplastic pulmonary arteries, and major aortopulmonary collateral vessels remains a challenge. The conventional surgical approach is a systemic-to-pulmonary artery shunt, which, in low-weight neonates, may imply major complications, such as shunt obstruction or thrombosis, pulmonary hyperflow, and infection.1 Another surgical option is partial correction, which requires the use of extracorporeal circulation and can lead to greater morbidity and mortality.
In the cardiac catheterization laboratory, radiofrequency perforation of the pulmonary valve with stent implantation in the right ventricular outflow tract has become a valid option for treating patients with pulmonary atresia, although it is associated with a need for follow-up surgery in 33% to 75% of patients.3 The most commonly described complications related to this technique are perforation of the cardiac wall and fracture or dislocation of the stent, among others.4
The hybrid procedure approach averts the need for extracorporeal circulation and is safer and faster than an interventional procedure, as it allows adequate anatomical inspection and enables the cardiovascular surgeon to act promptly if there are incidents. Other advantages are a shorter fluoroscopy time and no weight limitations in neonatal patients.5 Therefore, it can be concluded that valve perforation with stent implantation through the transventricular route using a median sternotomy is an effective palliative technique, especially for low-weight premature infants, that offers results similar to those of other therapeutic strategies with lower in-hospital morbidity and mortality rates and satisfactory long-term survival (). This technique achieves adequate development of the pulmonary arterial tree, so that corrective surgery can be performed in a second stage when the patient is older and has gained weight.6