To study the prevalence of poorly controlled vitamin K antagonist anticoagulation in Spain in patients with nonvalvular atrial fibrillation, and to identify associated factors.
MethodsWe studied 1056 consecutive patients seen at 120 cardiology clinics in Spain between November 2013 and March 2014. We analyzed the international normalized ratio from the 6 months prior to the patient's visit, calculating the prevalence of poorly controlled anticoagulation, defined as < 65% time in therapeutic range using the Rosendaal method.
ResultsMean age was 73.6 years (standard deviation, 9.8 years); women accounted for 42% of patients. The prevalence of poorly controlled anticoagulation was 47.3%. Mean time in therapeutic range was 63.8% (25.9%). The following factors were independently associated with poorly controlled anticoagulation: kidney disease (odds ratio = 1.53; 95% confidence interval, 1.08-2.18; P = .018), routine nonsteroidal anti-inflammatory drugs (odds ratio = 1.79; 95% confidence interval, 1.20-2.79; P = .004), antiplatelet therapy (odds ratio = 2.16; 95% confidence interval, 1.49-3.12; P < .0001) and absence of angiotensin receptor blockers (odds ratio = 1.39; 95% confidence interval, 1.08-1.79; P = .011).
ConclusionsThere is a high prevalence of poorly controlled vitamin K antagonist anticoagulation in Spain. Factors associated with poor control are kidney disease, routine nonsteroidal anti-inflammatory drugs, antiplatelet use, and absence of angiotensin receptor blockers.
Keywords
Nonvalvular atrial fibrillation (NVAF) is a prevalent condition. Its increasing incidence in Spain and neighboring countries is mainly due to the improved treatment of different cardiovascular diseases, which prolongs patients’ lives and contributes to an aging population. A recent epidemiological study in Spain, OFRECE, found that the prevalence of atrial fibrillation is 4.4% in adults older than 40 years, and that it increases exponentially with age.1 Atrial fibrillation has major implications because of its negative impact on survival, high incidence of embolic and bleeding events (especially stroke), the economic burden of treatment of the condition and its complications, and patients’ deteriorated quality of life.2 Current clinical practice guidelines for the management of patients with atrial fibrillation recommend preventive anticoagulation therapy in patients at risk of embolic events.2,3 Novel oral anticoagulants (OAC) that are direct thrombin or factor Xa inhibitors have been introduced in patients with NVAF.4–6 However, the classic vitamin K antagonists (VKA), such as coumarins and warfarin sodium, remain prevalent in Spain. This treatment reduces the risk of thromboembolic events, but its effectiveness depends on an adequate control of anticoagulation levels (defined as an international normalized ratio [INR] between 2 and 3). Several studies show that a high proportion of patients with NVAF receiving VKA may have an out-of-therapeutic-range INR for much of the time,7,8 which would place them at risk of thromboembolic and/or bleeding events. The aim of this study was to analyze the prevalence of poorly controlled VKA anticoagulation in patients with NVAF in Spain, and to identify factors associated with this poor control.
METHODSTo achieve this aim, the Research Agency of the Spanish Society of Cardiology designed the CALIFA study (Spanish acronym for Quality of anticoagulation and associated comorbidities in patients with nonvalvular Atrial Fibrillation at cardiology clinics). CALIFA was a national, multicenter, observational, cross-sectional, retrospective study of patients with NVAF receiving oral anticoagulation with VKA at cardiology clinics in Spain. Patients were consecutively enrolled by 120 cardiologists at outpatient cardiology clinics nationwide. Each cardiology investigator enrolled the first 10 patients from the study start date (November 1, 2013) who met all inclusion criteria and no exclusion criteria. Inclusion criteria were male or female patients aged 18 or older, with NVAF (defined as atrial fibrillation in patients without a valvular prosthesis, rheumatic mitral stenosis of any degree or significant, moderate or severe mitral regurgitation) taking a stable dose of VKA for at least 6 months before enrolment, who had granted their signed informed consent. Patients were excluded if their anticoagulant dose adjustment period had started during this 6-month period or if their VKA dose was changed or temporarily discontinued during the same period due to diagnostic or therapeutic interventions or procedures with a bleeding risk. All types of NVAF were permitted, including permanent, persistent, paroxysmal, and new-onset NVAF. This study was approved by the Research Ethics Committee of Hospital Universitario de San Juan de Alicante. As with all research involving human subjects, the study was carried out in accordance with the ethical principles of the Declaration of Helsinki and subsequent amendments. The study complied with the Spanish Data Protection Law.
The study enrolment period was 5 months (November 2013 to March 2014). Each patient attended a single visit. At this visit, the investigator collected data on the study variables from the previous 6 months. All INR test results from the same period were also collected, in order to calculate the time in therapeutic range (TTR) using the method of Rosendaal et al.9 In short, this method is based on the assumption that the INR value undergoes a broadly linear change when 2 determinations are measured over a given number of days, with the INR being interpolated between the 2 values, ie, the value increases or decreases by the same amount each day. For example, if the INR increases from 1.9 to 3.1 in a 14-day period, the difference of 1.2 points is assumed to change in a linear way over those 14 days (each day, the INR increases by 1.2/14 = 0.085). We used a program to automatically calculate the TTR. The primary study variable was the TTR of the INR, determined using the Rosendaal method. We defined poor anticoagulation control as a TTR of < 65% during the 6 months before study enrolment.
To make the sample as representative as possible, investigators’ clinics were widely distributed across Spain (Appendix 1 of the supplementary material). Table 1 shows the variables that were recorded in all patients. These variables were collected through a personal interview held with the patient at the enrolment visit and from patients’ medical records. The cardiologist was responsible for collecting this information. available online provides definitions of each variable. We calculated the sample size required to identify the factors associated with poor anticoagulation quality by assuming that 50% of patients would have poorly controlled anticoagulation (TTR < 65%) and that 1 in 4 patients would have the variables under study (eg, diabetes mellitus, kidney disease). The probability of a Type I error was set at 0.05 and a Type II error at 0.20. To estimate the relative risk of an out-of-range INR for a certain variable (eg, having diabetes mellitus vs not having it), 296 patients would be needed in the group with that variable (eg, diabetes) and 888 in the group without that variable (no diabetes) to detect a minimum relative risk of 1.20 if the rate of patients in the exposed group (out-of-range patients) was 50%. Assuming a patient loss rate of less than 10%, we estimated that a sample size of 1184 patients would be necessary. Finally, we were able to analyze 1056 patients who met the minimum requirement to calculate the TTR using the Rosendaal method (at least 4 INR determinations in the 6 months prior to enrolment), and therefore our estimated patient loss rate was valid.
Variables Collected and Analyzed in the CALIFA Study
| Demographic data |
| Age |
| Sex |
| Employment status |
| Social status |
| Level of education |
| Comorbidities and cardiovascular risk factors |
| Hypertension |
| Hyperlipidemia |
| Diabetes mellitus |
| Smoking status |
| Chronic obstructive pulmonary disease |
| Kidney disease |
| Liver disorder |
| History of cancer |
| Aortic disease or lower limb arterial disease |
| Previous stroke |
| Previous non-cerebral embolism |
| Routine nonsteroidal anti-inflammatory drugs |
| Thyroid dysfunction |
| Alcohol or drug abuse |
| Dementia |
| Cardiology history, apart from AF |
| Drugs that increase bleeding (nonsteroidal anti-inflammatory drugs, antiplatelets) |
| Significant kidney disease (glomerular filtration rate < 60 mL/min) |
| Severe kidney disease (dialysis, kidney transplantation, glomerular filtration rate < 30 mL/min or serum creatinine ≥ 2.2 mg/dL) |
| Previous heart disease, and type |
| Previous tachyarrhythmias |
| Previous bradyarrhythmias |
| Previous ablation |
| Pacemaker |
| Biventricular pacemaker / implantable cardioverter-defibrillator; |
| Modified Charlson comorbidity index |
| Bleeding history |
| Atrial fibrillation data |
| Time since initial diagnosis |
| Type of atrial fibrillation |
| Previous electrical cardioversion |
| Previous ablation |
| Rhythm or rate control strategy |
| Previous atrial appendage closure |
| EHRA functional class |
| CHADS2 |
| CHA2DS2-VASc |
| HAS-BLED |
| Examination and diagnostic tests |
| Systolic Blood Pressure |
| Diastolic blood pressure |
| Heart rate |
| Weight |
| Height |
| Body mass index |
| Waist circumference |
| Rhythm detected on ECG |
| Conduction disorder detected on ECG |
| Left ventricular ejection fraction (most recent in past 6 months) |
| Blood test (most recent in past 6 months) |
| Hemoglobin |
| Fasting glucose |
| Triglycerides |
| Serum creatinine |
| Total cholesterol |
| Glomerular filtration rate |
| HDL-C |
| HbA1c |
| Drug treatment in past 6 months |
| Vitamin K antagonists |
| Mineralocorticoid receptor antagonists |
| Angiotensin-converting enzyme inhibitors |
| Angiotensin receptor blockers |
| Diuretics |
| Statins |
| Antiplatelets |
| Beta-blockers |
| Digoxin |
| Calcium antagonists |
| Antiarrhythmics |
| INR test results in past 6 months |
| Approach to anticoagulant treatment |
AF, atrial fibrillation; ECG, electrocardiogram; EHRA, European Heart Rhythm Association; HbA1c, glycated hemoglobin; HDL-C, cholesterol-associated high density lipoprotein; INR, International Normalized Ratio.
Using the Rosendaal method, we expressed the prevalence of poorly controlled anticoagulation as the percentage of all patients analyzed who had a TTR < 65%. We asked the investigators whether they would make any change in anticoagulation treatment after seeing their patient's TTR score. The 4 possible responses were: continue as before; switch to a novel OAC; improve VKA; discontinue the OAC. Only 1 response could be selected for each patient. We used chi-square and the Mann-Whitney test to perform a bivariate comparative analysis of all variables related to history, comorbidities, and drug treatment collected in the subgroups of patients with TTR ≥ or < 65%. Statistical significance was defined as P < .05. Factors showing statistical significance and those with P ≤ .15 were entered into a multivariate logistic regression model, and we estimated their odds ratio (OR) and 95% confidence intervals (95%CI).
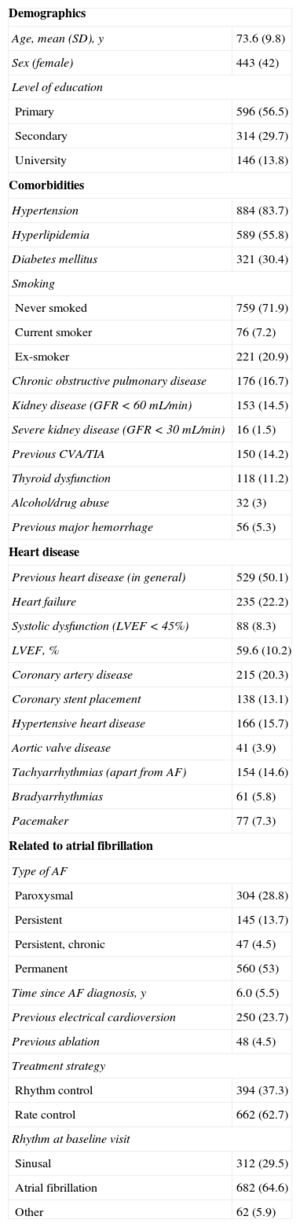
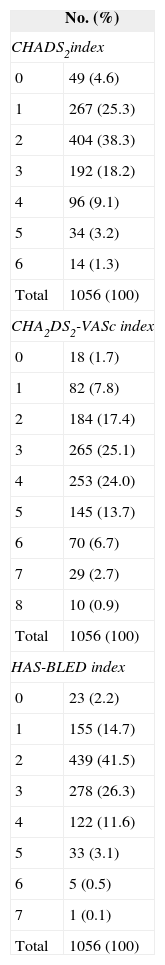
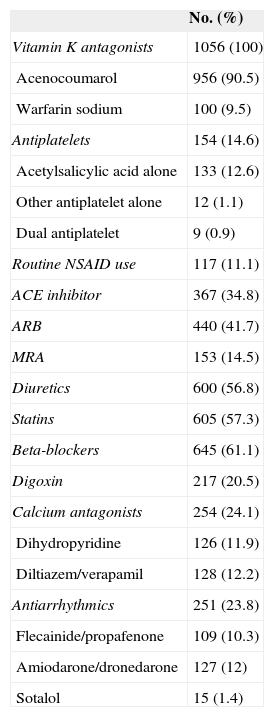
RESULTSGeneral Characteristics of the SampleMean age was 73.6 (SD, 9.8) years; 42% were women. Table 2 shows the most important demographic and medical data in our patients. The mean scores for the CHADS2 and the CHA2DS2-VASc indices were 2.2 (SD, 1.2) (range, 0-6) and 3.5 (1.6) (range, 0-8), respectively. The mean HAS-BLED score was 2.4 (SD, 1.1) (range, 0-7). Table 3 shows distribution by these scores. All patients received VKA for at least 6 months before enrolment. Acenocoumarol was prescribed in 90.5% and warfarin sodium in 9.5%. Table 4 details the drug treatment received by patients during the 6 months before enrolment.
Medical History and General Characteristics (N = 1056)
| Demographics | |
| Age, mean (SD), y | 73.6 (9.8) |
| Sex (female) | 443 (42) |
| Level of education | |
| Primary | 596 (56.5) |
| Secondary | 314 (29.7) |
| University | 146 (13.8) |
| Comorbidities | |
| Hypertension | 884 (83.7) |
| Hyperlipidemia | 589 (55.8) |
| Diabetes mellitus | 321 (30.4) |
| Smoking | |
| Never smoked | 759 (71.9) |
| Current smoker | 76 (7.2) |
| Ex-smoker | 221 (20.9) |
| Chronic obstructive pulmonary disease | 176 (16.7) |
| Kidney disease (GFR < 60 mL/min) | 153 (14.5) |
| Severe kidney disease (GFR < 30 mL/min) | 16 (1.5) |
| Previous CVA/TIA | 150 (14.2) |
| Thyroid dysfunction | 118 (11.2) |
| Alcohol/drug abuse | 32 (3) |
| Previous major hemorrhage | 56 (5.3) |
| Heart disease | |
| Previous heart disease (in general) | 529 (50.1) |
| Heart failure | 235 (22.2) |
| Systolic dysfunction (LVEF < 45%) | 88 (8.3) |
| LVEF, % | 59.6 (10.2) |
| Coronary artery disease | 215 (20.3) |
| Coronary stent placement | 138 (13.1) |
| Hypertensive heart disease | 166 (15.7) |
| Aortic valve disease | 41 (3.9) |
| Tachyarrhythmias (apart from AF) | 154 (14.6) |
| Bradyarrhythmias | 61 (5.8) |
| Pacemaker | 77 (7.3) |
| Related to atrial fibrillation | |
| Type of AF | |
| Paroxysmal | 304 (28.8) |
| Persistent | 145 (13.7) |
| Persistent, chronic | 47 (4.5) |
| Permanent | 560 (53) |
| Time since AF diagnosis, y | 6.0 (5.5) |
| Previous electrical cardioversion | 250 (23.7) |
| Previous ablation | 48 (4.5) |
| Treatment strategy | |
| Rhythm control | 394 (37.3) |
| Rate control | 662 (62.7) |
| Rhythm at baseline visit | |
| Sinusal | 312 (29.5) |
| Atrial fibrillation | 682 (64.6) |
| Other | 62 (5.9) |
AF, atrial fibrillation; CVA, cerebrovascular accident; GFR, glomerular filtration rate; LVEF, left ventricular ejection fraction; SD, standard deviation; TIA, transient ischemic attack.
Quantitative variables are expressed as mean (standard deviation) and qualitative variables as No. (%).
Case Distribution by Thromboembolic and Bleeding Risk Index Score
| No. (%) | |
|---|---|
| CHADS2index | |
| 0 | 49 (4.6) |
| 1 | 267 (25.3) |
| 2 | 404 (38.3) |
| 3 | 192 (18.2) |
| 4 | 96 (9.1) |
| 5 | 34 (3.2) |
| 6 | 14 (1.3) |
| Total | 1056 (100) |
| CHA2DS2-VASc index | |
| 0 | 18 (1.7) |
| 1 | 82 (7.8) |
| 2 | 184 (17.4) |
| 3 | 265 (25.1) |
| 4 | 253 (24.0) |
| 5 | 145 (13.7) |
| 6 | 70 (6.7) |
| 7 | 29 (2.7) |
| 8 | 10 (0.9) |
| Total | 1056 (100) |
| HAS-BLED index | |
| 0 | 23 (2.2) |
| 1 | 155 (14.7) |
| 2 | 439 (41.5) |
| 3 | 278 (26.3) |
| 4 | 122 (11.6) |
| 5 | 33 (3.1) |
| 6 | 5 (0.5) |
| 7 | 1 (0.1) |
| Total | 1056 (100) |
Drug Treatment in the Past 6 Months (N = 1056)
| No. (%) | |
|---|---|
| Vitamin K antagonists | 1056 (100) |
| Acenocoumarol | 956 (90.5) |
| Warfarin sodium | 100 (9.5) |
| Antiplatelets | 154 (14.6) |
| Acetylsalicylic acid alone | 133 (12.6) |
| Other antiplatelet alone | 12 (1.1) |
| Dual antiplatelet | 9 (0.9) |
| Routine NSAID use | 117 (11.1) |
| ACE inhibitor | 367 (34.8) |
| ARB | 440 (41.7) |
| MRA | 153 (14.5) |
| Diuretics | 600 (56.8) |
| Statins | 605 (57.3) |
| Beta-blockers | 645 (61.1) |
| Digoxin | 217 (20.5) |
| Calcium antagonists | 254 (24.1) |
| Dihydropyridine | 126 (11.9) |
| Diltiazem/verapamil | 128 (12.2) |
| Antiarrhythmics | 251 (23.8) |
| Flecainide/propafenone | 109 (10.3) |
| Amiodarone/dronedarone | 127 (12) |
| Sotalol | 15 (1.4) |
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blockers; MRA, mineralocorticoid receptor antagonists; NSAID, nonsteroidal anti-inflammatory drugs.
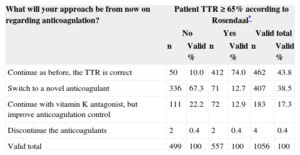
A total of 6758 INR determinations were recorded among the 1056 patients in the 6-month period analyzed (6.40 determinations per patient and 1.07 per patient/month). Only 59.1% (24.8%) of determinations were out-of-therapeutic range (INR, 2–3). This figure was similar nationwide. The distribution of patients by number of in-range determinations was as follows: 13.6% of patients had < 30% of determinations in range; 30% of patients had 30%-50% in range; 21.7% had 50%-70% in range, 34.7% of patients had > 70% in range. According to the Rosendaal method, the mean TTR for our sample during this period was 63.8 (25.9%). The prevalence of poorly controlled anticoagulation, defined as a TTR < 65% according to the Rosendaal method, was 47.3% (95%CI, 44.3%-50.3%; n = 499). Of all patients, 29.6% had a TTR of < 50%. Table 5 shows the investigators’ approach to anticoagulant therapy, depending on whether the TTR was higher or lower than 65%. Of all investigators, 67.3% said they would switch to a novel anticoagulant if the TTR was < 65%, compared with just 12.7% who would do so with a TTR ≥ 65% (P < .001).
Investigators’ Approach Regarding Anticoagulation Therapy According to Time in Therapeutic Range
| What will your approach be from now on regarding anticoagulation? | Patient TTR ≥ 65% according to Rosendaal* | |||||
|---|---|---|---|---|---|---|
| No | Yes | Valid total | ||||
| n | Valid % | n | Valid % | n | Valid % | |
| Continue as before, the TTR is correct | 50 | 10.0 | 412 | 74.0 | 462 | 43.8 |
| Switch to a novel anticoagulant | 336 | 67.3 | 71 | 12.7 | 407 | 38.5 |
| Continue with vitamin K antagonist, but improve anticoagulation control | 111 | 22.2 | 72 | 12.9 | 183 | 17.3 |
| Discontinue the anticoagulants | 2 | 0.4 | 2 | 0.4 | 4 | 0.4 |
| Valid total | 499 | 100 | 557 | 100 | 1056 | 100 |
TTR, time in therapeutic range.
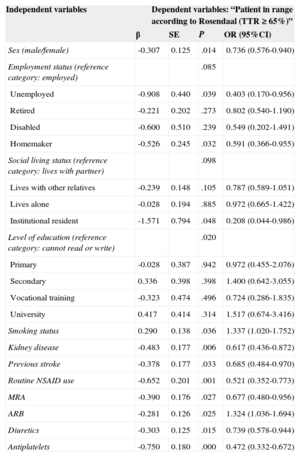
Table 6 shows the results of the bivariate comparison of the different variables analyzed in the 499 patients with TTR < 65% and the 557 with TTR ≥ 65%. In the group with TTR < 65% when the Rosendaal method was used, there were more women (45.9% vs 38.4% in the other group, P = 0.014), unemployed (3.4% vs 1.8%, P = .039), homemakers (17.4% vs 13.5% P = .032), institution residents (1.6% vs 0.4%, P = .048), patients with kidney disease (glomerular filtration rate < 60mL/min) (17.6% vs 11.7%, P = .006), previous stroke (16.6% vs 12%, P = .033), and routine nonsteroidal anti-inflammatory drug use (14.4% vs 8.1%, P = .001). This group also had a lower rate of past or present smoking than the group with TTR ≥ 65% (25.1% vs 30.9%, P = .036). With regard to drug treatment, diuretics, aldosterone antagonists, and antiplatelets were more common in patients with TTR < 65%, while angiotensin receptor blockers were more common in patients with TTR ≥ 65% (Table 6). There were no significant differences for the other variables analyzed.
Significant Variables in the Bivariate Comparison Between the 499 Patients with TTR < 65% and the 557 Patients With TTR ≥ 65%
| Independent variables | Dependent variables: “Patient in range according to Rosendaal (TTR ≥ 65%)” | |||
|---|---|---|---|---|
| β | SE | P | OR (95%CI) | |
| Sex (male/female) | -0.307 | 0.125 | .014 | 0.736 (0.576-0.940) |
| Employment status (reference category: employed) | .085 | |||
| Unemployed | -0.908 | 0.440 | .039 | 0.403 (0.170-0.956) |
| Retired | -0.221 | 0.202 | .273 | 0.802 (0.540-1.190) |
| Disabled | -0.600 | 0.510 | .239 | 0.549 (0.202-1.491) |
| Homemaker | -0.526 | 0.245 | .032 | 0.591 (0.366-0.955) |
| Social living status (reference category: lives with partner) | .098 | |||
| Lives with other relatives | -0.239 | 0.148 | .105 | 0.787 (0.589-1.051) |
| Lives alone | -0.028 | 0.194 | .885 | 0.972 (0.665-1.422) |
| Institutional resident | -1.571 | 0.794 | .048 | 0.208 (0.044-0.986) |
| Level of education (reference category: cannot read or write) | .020 | |||
| Primary | -0.028 | 0.387 | .942 | 0.972 (0.455-2.076) |
| Secondary | 0.336 | 0.398 | .398 | 1.400 (0.642-3.055) |
| Vocational training | -0.323 | 0.474 | .496 | 0.724 (0.286-1.835) |
| University | 0.417 | 0.414 | .314 | 1.517 (0.674-3.416) |
| Smoking status | 0.290 | 0.138 | .036 | 1.337 (1.020-1.752) |
| Kidney disease | -0.483 | 0.177 | .006 | 0.617 (0.436-0.872) |
| Previous stroke | -0.378 | 0.177 | .033 | 0.685 (0.484-0.970) |
| Routine NSAID use | -0.652 | 0.201 | .001 | 0.521 (0.352-0.773) |
| MRA | -0.390 | 0.176 | .027 | 0.677 (0.480-0.956) |
| ARB | -0.281 | 0.126 | .025 | 1.324 (1.036-1.694) |
| Diuretics | -0.303 | 0.125 | .015 | 0.739 (0.578-0.944) |
| Antiplatelets | -0.750 | 0.180 | .000 | 0.472 (0.332-0.672) |
95%CI, 95% confidence interval; ARB, angiotensin receptor blockers; MRA, mineralocorticoid receptor antagonists; NSAID, nonsteroidal anti-inflammatory drugs; OR, odds ratio; SE, standard error; TTR, time in therapeutic range.
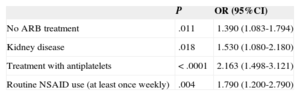
In the multivariate study (Table 7), 4 variables were independently associated with a TTR < 65%: moderate or severe kidney disease (OR = 1.53; 95%CI, 1.08-2.18; P = .018), routine nonsteroidal anti-inflammatory drugs (OR = 1.79; 95%CI, 1.20-2.79; P = .004), antiplatelet therapy (OR = 2.16; 95%CI, 1.49-3.12; P < .0001) and absence of angiotensin receptor blockers (OR = 1.39; 95%CI, 1.08-1.79; P = .011).
Significant Variables Associated With Poorly Controlled VKA Anticoagulation (TTR < 65%) in the Multivariate Analysis
| P | OR (95%CI) | |
|---|---|---|
| No ARB treatment | .011 | 1.390 (1.083-1.794) |
| Kidney disease | .018 | 1.530 (1.080-2.180) |
| Treatment with antiplatelets | < .0001 | 2.163 (1.498-3.121) |
| Routine NSAID use (at least once weekly) | .004 | 1.790 (1.200-2.790) |
95%CI, 95% confidence interval; ARB, angiotensin receptor blockers; NSAID, nonsteroidal anti-inflammatory drugs; OR, odds ratio; TTR, time in therapeutic range; VKA, vitamin K antagonists.
The CALIFA study provides an ample, representative sample of patients with NVAF receiving VKA in Spain today. The study results show that there is poor quality and poor control of VKA anticoagulation in this country. Almost half the patients (47.3%) were out-of-therapeutic range more than 65% of the time (TTR < 65%), calculated with the method of Rosendaal et al9, currently the most widely accepted method. Moreover, almost a third of patients treated with VKA were out-of-range for more than half the time (TTR < 50%). This means that half the patients with NVAF and a high embolic risk have a high probability of thromboembolic events, despite being theoretically protected by the anticoagulation therapy. Poor control of the degree of anticoagulation is one of the strongest independent predictors of thromboembolic and bleeding complications.10–12
Previous studies reported a high prevalence (about 30%-40%) of poorly controlled anticoagulation with VKA, and this figure does not appear to have decreased in the last 20 years.7,8,13–15 Although the prevalence of poorly controlled anticoagulation is higher in clinical registers that include patients in real-life practice,7 in more recent clinical trials, such as RE-LY8 and ROCKET AF,10 between 30% and 40% of patients with NVAF receiving VKA are still out of range over 60% to 65% of the time, with an INR < 2 or > 3. Our study results are similar or even worse, because almost 50% of patients had a TTR < 65%. Also, the CALIFA study only included patients with a “stable” VKA anticoagulation regimen, thus excluding those who had started anticoagulation during the previous 6 months and those whose anticoagulation had been temporarily discontinued or whose VKA dose had been changed due to procedures with a bleeding risk. If we had included these patients, who are commonly seen in daily practice, the prevalence of poor control would probably have been even higher. Furthermore, by defining correct anticoagulation as a TTR of > 65%, we are assuming that these patients may have an out-of-range INR for up to 35% of the time, which is a risk in itself. However, the Spanish health authorities use the 65% cut-off point in patients receiving VKA to indicate the use of novel OACs in patients with NVAF.16 To be coherent, we chose the same cut-off point for our analysis.
The second objective of our study was to identify possible predictors of VKA OAC quality, because these predictors could have a major impact when a decision must be made deciding on OAC treatment strategy in patients with this indication. Previous studies have found different variables and comorbidities to be associated with worse VKA OAC control,17–21 although there is considerable variability among the studies. Younger age and female sex are independent predictors of poor OAC control according to some studies,17 but not others.18 This variability is also observed in level of education and language proficiency.18,19 Some studies have found the following factors to be predictors of poor quality VKA anticoagulation: diet (food with high vitamin K content, alcohol consumption), body mass index, non-Caucasian race, comorbidities (diabetes mellitus, previous stroke, heart failure) and concomitant drug treatments (nonsteroidal anti-inflammatory drugs, amiodarone).17–21 However, other drugs, such as beta-blockers and verapamil, have been associated with improved VKA OAC control.17 With regard to smoking status, one study found that smoking in the previous 2 years is a very strong predictor of poor OAC control,17 and another observed that never having smoked was associated with a higher risk of INR > 3.18 The association with race may be due to level of education19 or genetic differences in genes involved in vitamin K or cytochrome p450 metabolism.21 In our study, we found 4 variables were independent predictors of poorly controlled VKA anticoagulation: 1 comorbidity factor (kidney disease with glomerular filtration rate < 60mL/min) and 3 drug factors. Routine use of nonsteroidal anti-inflammatory drugs or antiplatelets was associated with poor anticoagulation control, while angiotensin receptor blockers were associated with good control. The association between VKA and OAC control and these drugs (and beta-blockers, verapamil, and amiodarone described in other studies)17 may be explained by pharmacokinetic interactions in metabolic degradation mechanisms.
The variability and discrepancy among results that point to possible predictors of good or poor OAC control with VKA make it hard to identify which patients will have poor anticoagulation control with VKA. Some authors have tried to simplify this problem by applying indices that incorporate weighed individual factors. One such index is SAMe-TT2R2 (female sex, age < 60 years, at least 2 comorbidities, treatment with rhythm control, tobacco and race), proposed by Apostolakis et al, based on the AFFIRM study cohort.17 Although some recent studies appear to validate this index,22,23 more experience may be necessary before it can be adopted as the guideline of choice when a decision is made on treatment with a VKA or other OACs.
Our study results show a high prevalence of poor anticoagulation among patients with NVAF who are currently receiving VKA in Spain. This situation has remained unchanged for the past 20 years. These theoretically “protected” patients are therefore at a high risk of stroke, repeat embolisms and bleeding complications. Strategies are needed to improve this situation. Different alternatives have been proposed: one strategy proposes the use of VKAs with greater anticoagulant stability, such as warfarin sodium or phenprocoumon24 (a coumarin with a longer half-life than acenocoumarol), but the latter is not marketed in Spain. In our study, almost 10% of patients received warfarin, and the results were similar to those of acenocoumarol. Paradoxically, the addition of a daily vitamin K supplement can increase VKA anticoagulation stability,25 although there is little experience with this regimen. Other alternatives are strategies involving specialized anticoagulation clinics or patient self-management,26 but the latter can only be applied in carefully selected patients. Two very recent studies have suggested using VKA dose adjustment regimens with algorithms that take into consideration the genes involved in VKA metabolism (VKORC1 and CYP2C9),27,28 because it has been shown that polymorphisms in these genes may account for a third of the variations in VKA dosage requirements. However, this is a complex alternative, and, in addition, these 2 controlled studies found contradictive results (favorable for warfarin27 and unfavorable for acenocoumarol).28 Finally, possibly the most effective, safe and feasible alternative4–6 is to switch poorly coagulated patients to novel OACs. Indeed, this option was preferred by most of the investigators in our study.
LimitationsThe main limitations of our study stem from its nonrandomized, observational design. Also, potential bias from nonrandomized patient enrolment means that we cannot rule out a tendency to include poorly controlled patients. However, this potential enrolment bias is reduced by the large sample size, short enrolment period, and nationwide distribution of investigators. This study provides up-to-date results from a “real life” Spanish population.
CONCLUSIONSOur results show a high prevalence (almost 50%) of poor anticoagulation control among Spanish patients with NVAF who are currently receiving a stable dose of VKA. Factors that are independently associated with this poor control are kidney disease and routine use of nonsteroidal anti-inflammatory drugs and antiplatelets. Angiotensin receptor blockers are associated with good anticoagulation control. These results show that a high proportion of theoretically “protected” patients with NVAF are at a high risk of thromboembolic and bleeding events, and that we need strategies to improve this situation. These strategies should aim to identify the factors associated with poor anticoagulation with VKA, improve the management of VKA control, and increase the use of novel OACs where indicated.
FUNDINGThis study was funded by an unrestricted research grant from Bayer Hispania S.L.
CONFLICTS OF INTERESTNone declared.