Despite the availability of primary and secondary prevention strategies,1,2 antithrombotic therapies with a prompt mechanism of action3,4 and rapid coronary reperfusion strategies,5,6 there are still a considerable number of patients presenting with acute coronary syndrome (ACS) who are affected by hemodynamic (ie, advanced Killip class) or electrical (i.e, out-of-hospital cardiac arrest) instability.7 These cohorts of ACS participants with these dreaded complications can be grouped under the generic definition of “vulnerable patients” (VP). Recent data show that the incidence of VP ranges from 3% to 13% of ACS patients, with a prevalence that is 2- to 3- fold higher among those presenting with an ST-segment elevation myocardial infarction compared with a non–ST−segment elevation myocardial infarction.7 Importantly, VP are characterized by very high mortality rates, underscoring the need for strategies to improve their prognosis.7 Unfortunately, VP are very often excluded from randomized controlled trials (RCTs), precluding definition of the best evidenced-based approach for their management. This includes the vascular access site to be used and the optimal antithrombotic therapy, both key aspects in the care of this highly complex patient population when undergoing percutaneous coronary interventions (PCI).
The use of radial vascular access site is nowadays universally accepted as the safest and preferred access site in patients undergoing PCI.5,6 However, in VP, femoral access is more commonly used than radial access.8 This may be attributed to the perception that femoral access may be easier in this setting (eg, due to the femoral pulse being more palpable than the radial pulse if blood pressure is low) or that these patients may require more complex coronary intervention, hence possibly requiring larger bore access.9 Nevertheless, radial artery access and the ability to perform complex coronary interventions via this route largely depend on operator expertise. It should also be noted that the use of femoral access in VP, which is often not ultrasound-guided, is associated with an enhanced risk of access site complications, bleeding, and death.8,9
Recent ACS guidelines5,6 recommend the use of unfractionated heparin (UFH) as the standard of care for intraprocedural anticoagulation, although this recommendation is supported by low evidence (class I, level of evidence C). The pharmacodynamic and pharmacokinetic advantages of bivalirudin over UFH, which have translated into reduced bleeding complications, have allowed this drug to become a reasonable treatment option in ACS patients undergoing PCI.10 However, the clinical implications associated with the use of bivalirudin in a vulnerable cohort of patients, such as those with advanced Killip class or electrical instability, who are also at increased risk for bleeding complications, remains unknown.11
In a recent article published in Revista Española de Cardiología, Gargiulo et al.12 report the results of a post-hoc analysis of VP from the MATRIX (Minimizing Adverse Haemorrhagic Events by Transradial Access Site and Systemic Implementation of Angiox, NCT01433627) program comparing radial vs femoral vascular access and bivalirudin vs UFH. In brief, MATRIX was a multicenter, prospective, open-label, trial conducted in ACS patients who were randomized in a 2 x 2 factorial fashion to transradial (n=4197) vs transfemoral (n=4207) intervention (MATRIX-Access) and intraprocedural bivalirudin (n=3610) vs UFH (n=3603) plus provisional use of glycoprotein IIb/IIIa inhibitors (MATRIX-Antithrombin). A third randomization among patients treated with bivalirudin compared postprocedure infusion (n=1799) with no postprocedure infusion (n=1811) of bivalirudin (MATRIX-Treatment Duration). However, outcomes based on this comparison were not included in this post-hoc analysis. The coprimary outcomes for MATRIX-Access and MATRIX-Antithrombin were major adverse cardiovascular events (MACE), defined as the composite of all-cause mortality, myocardial infarction, and stroke up to 30 days and net adverse clinical events (NACE), defined as the composite of noncoronary artery bypass graft-related major bleeding (Bleeding Academic Research Consortium [BARC] type 3 or 5) or MACE up to 30 days. The trial outcomes at 30 days and 1 year have been previously reported.13–15 In both MATRIX-Access and MATRIX-Antithrombin, the 2 coprimary composite endpoints of MACE and NACE did not differ,14 except that NACE was significantly less frequent with radial than with femoral access, driven by lower, albeit not statistically significant, BARC major bleeding and all-cause mortality.13 Although there were no differences in the individual secondary outcomes of any BARC and BARC major bleeding, all-cause and cardiovascular mortality irrespective of vascular access, these were significantly reduced with bivalirudin use compared with UFH. However, bivalirudin use was associated with a significant increase in the rate of definite stent thrombosis.
A total of 934 patients in MATRIX-Access and MATRIX-Antithrombin, representing approximately 11.1% of the trial population, were defined as VP and included in this post-hoc analysis. Specifically, 472 (5.5%) were allocated to radial and 472 (5.6%) to femoral access and 397 (5.5%) were allocated to bivalirudin and 422 (5.0%) to UFH. To the best of our knowledge, this cohort of VP represents the largest and most contemporary assessment of outcomes according to vascular access site and antithrombotic treatment regimen.16 The authors should be commended for including these most critically ill patients in their trial, allowing the identification of insights on the best management for these patients, who are often excluded from RCTs. Inclusion of these patients also speaks to the real-world nature of the MATRIX trial experience, showing rates of VP reflective of real-world practice. Despite the inherent limitations of similar post-hoc analyses, correctly acknowledged by the authors, those performed in VP patients are indeed highly informative and clinically useful.
As could be expected, the VP population was much sicker and at higher risk than the non-VP population. The use of glycoprotein IIb/IIIa inhibitors, intra-aortic balloon pump, the presence of left main or multivessel disease, and need for coronary artery bypass grafting were more common among VP. At 30 days, VP had significantly higher rates of nearly all ischemic (MACE, NACE, all-cause and cardiovascular mortality, stroke, stent thrombosis, urgent target vessel revascularization) and bleeding (any and major bleeding according to various classifications) outcomes compared with non-VP. Although baseline and procedural characteristics were generally well matched in VP and non-VP subgroups allocated to radial vs femoral or bivalirudin vs UFH treatments, VP were significantly older according to the randomized antithrombin type and had significantly more ST-segment elevation myocardial infarction presentation according to the randomized access site.
In this analysis, no significant interaction for the primary or secondary endpoints was shown between access site (radial vs femoral) or intraprocedural anticoagulation (bivalirudin vs UFH) and VP criteria, with the sole exception of BARC 3a bleeding favoring bivalirudin in VP. In the VP cohort, radial compared with femoral vascular access site conferred a significant reduction of any BARC (relative risk [RR], 0.64; 95% confidence interval [95%CI]), 0.44-0.90) and BARC major bleeding (RR, 0.47; 95%CI, 0.24-0-95), with a trend toward reduced MACE (RR, 0.89; 95%CI, 0.64-1.25), NACE (RR, 0.82; 95%CI, 0.59-1.13), all-cause mortality (RR, 0.80; 95%CI, 0.51-1.25), and cardiovascular mortality (RR, 0.81; 95%CI, 0.51-1.28). Of interest, the benefits of the radial approach were significantly blunted in both VP and non-VP cohorts when centers with a low or average proportion of radial access PCI were included. In the VP cohort, bivalirudin compared with UFH conferred a significant reduction in BARC major bleeding (RR, 0.30; 95%CI, 0.13-0.63), all-cause mortality (RR, 0.51; 95%CI, 0.31-0.84) and cardiovascular mortality (RR, 0.50; 95%CI, 0.30-0.53), a trend toward reduced MACE (RR, 0.84; 95%CI, 0.59-1.19), NACE (RR, 0.73; 95%CI, 0.52-1.02), and any BARC bleeding (RR, 0.72; 95%CI, 0.50-1.02). However, bivalirudin was associated with a numerical increase in myocardial infarction (RR, 1.46; 95%CI, 0.88-2.41) and definite stent thrombosis (RR, 1.54; 95%CI, 0.55-4.35) in VP.
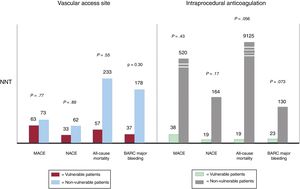
The major limitation of this study is its low statistical power to investigate the effects of experimental treatment strategies in VP. In fact, while the VP cohort is overall among the largest encountered in an ACS trial, in absolute terms it is still too small to allow any definitive conclusions to be drawn and the results should be considered as hypothesis-generating, particularly considering that the analysis was also post-hoc and that randomization was not stratified according to VP status. Moreover, in light of the above-mentioned different baseline and procedural characteristics among VP compared with non-VP allocated to radial vs femoral or bivalirudin vs UFH treatments, the lack of a multivariate regression analysis does not allow exclusion of independent predictors on the included outcomes. Despite these limitations and the low probability of a large RCT being designed to identify the optimal vascular access site and antithrombotic treatment regimen selectively in VP, we should make the best out of the data provided, which represents the largest, contemporary and reflective of real-word patients. In particular, these findings overall support, particularly in the hands of experienced operators, the choice of the radial artery over the femoral approach for vascular access and bivalirudin over UFH as antithrombotic regimen. The very high event rates among VP contribute to the increased absolute risk reduction with radial access or bivalirudin treatment compared with non-VP, as illustrated by the number needed to treat with regards to key outcomes, including the coprimary composite endpoints of MACE and NACE, all-cause mortality, and BARC major bleeding (figure 1).
Number needed to treat (NNT) according to vascular access site (radial vs femoral) or intraprocedural anticoagulation (bivalirudin vs unfractionated heparin) in vulnerable compared with nonvulnerable patients. BARC, Bleeding Academic Research Consortium; MACE, major adverse cardiovascular events; NACE, net adverse clinical events.
In conclusion, very limited data are available in the setting of ACS patients with hemodynamic or electrical instability and it is unlikely that RCTs will be performed to determine the optimal vascular access site and intraprocedural anticoagulation in these patients. While greater effort to include VP in prospective or matched case-control retrospective studies is advisable, this large post-hoc analysis from a relevant RCT on this topic supports the potential advantages of radial compared with femoral artery for vascular access, as long as it is used in centers with a high volume of radial access PCI, and of bivalirudin compared with UFH, which were consistent in non-VP and VP, with a greater absolute risk reduction in the latter related to a higher baseline ischemic and bleeding risk.
CONFLICTS OF INTERESTD.J. Angiolillo declares that he has received consulting fees or honoraria from Abbott, Amgen, Aralez, AstraZeneca, Bayer, Biosensors, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, Daiichi-Sankyo, Eli Lilly, Haemonetics, Janssen, Merck, PhaseBio, PLx Pharma, Pfizer, Sanofi, and The Medicines Company and has received payments for participation in review activities from CeloNova and St Jude Medical. D.J. Angiolillo also declares that his institution has received research grants from Amgen, AstraZeneca, Bayer, Biosensors, CeloNova, CSL Behring, Daiichi-Sankyo, Eisai, Eli Lilly, Gilead, Janssen, Matsutani Chemical Industry Co., Merck, Novartis, Osprey Medical, Renal Guard Solutions and Scott R. MacKenzie Foundation. M. Galli has nothing to disclose.