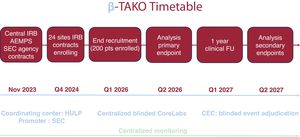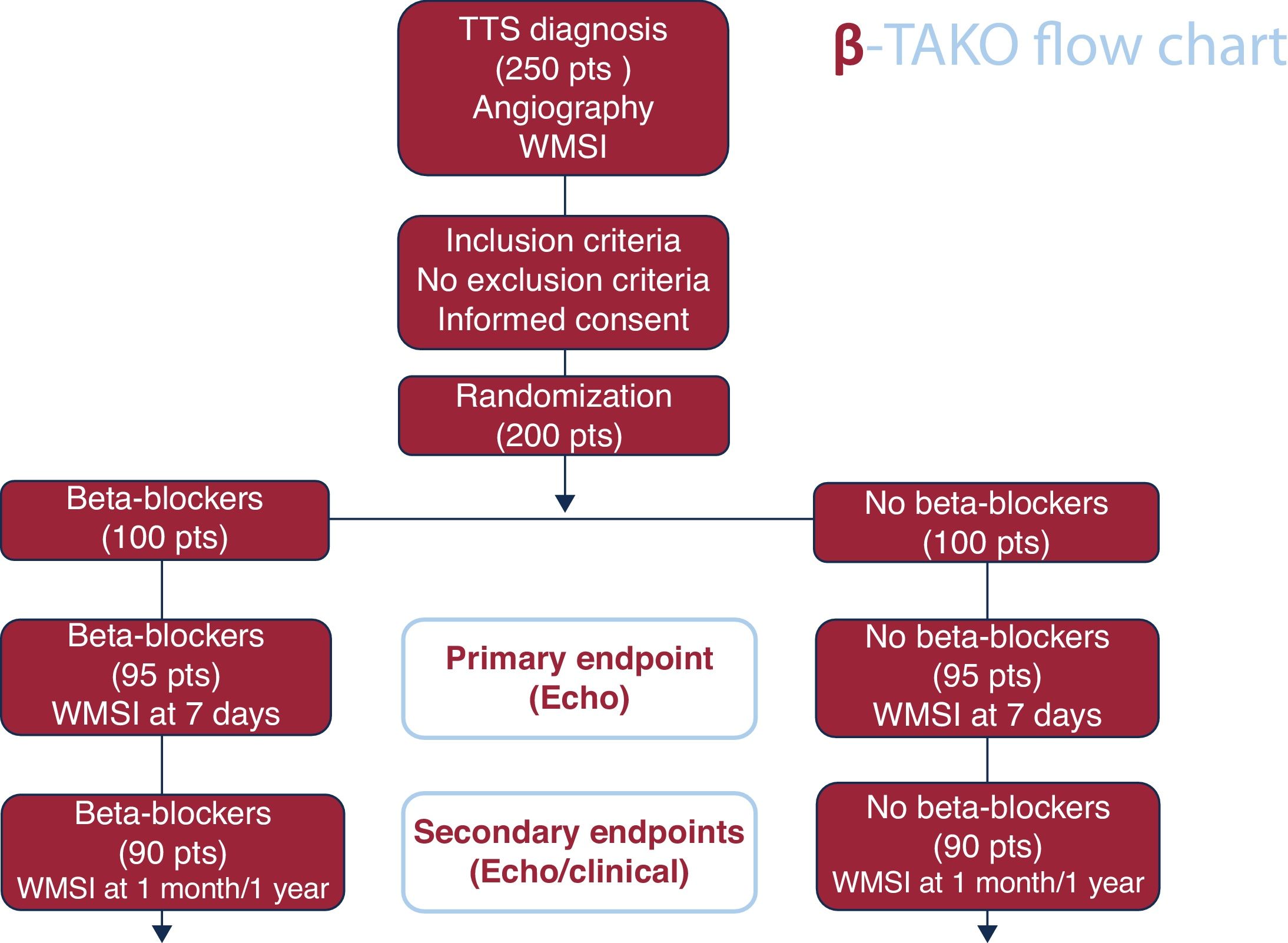
Tako-tsubo syndrome (TTS) is a cardiac condition that mimics acute coronary syndrome, characterized by transient left ventricular dysfunction in the absence of culprit coronary artery stenosis. Although its etiology remains unknown, reversible microvascular dysfunction secondary to an adrenergic surge is thought to play a role. Treatment is empirical, although most patients receive beta-blockers (BB) in clinical practice. The Beta-blockers in Tako-tsubo Syndrome study (β-Tako), is an academic, multicenter, pragmatic, prospective randomized open-label trial with blinded endpoint evaluation that aims to assess the efficacy and safety of BB in patients with TTS.
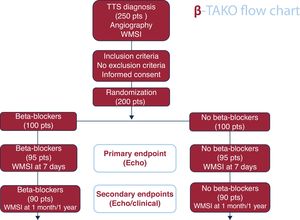
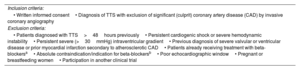
MethodsThe diagnosis of TTS will be confirmed by invasive coronary angiography and serial echocardiographic assessments. Two hundred patients with TTS will be randomized (1:1) to BB (n=100) or no BB (n=100). BB with alpha or nitric oxide release activity will be used in the treatment arm.
ResultsThe primary efficacy endpoint is the comparison of the wall motion score index by echocardiography at 7 days, analyzed by an independent core laboratory. Changes in left ventricular ejection fraction and global longitudinal strain will also be evaluated. A composite clinical endpoint (death, stroke, admission for recurrent TTS, acute coronary syndrome, heart failure, or atrial fibrillation) at 1 year will be assessed by an independent clinical events committee. Several predefined substudies will be conducted to examine clinical, imaging, biomarker, pharmacogenetic, inflammatory, messenger ribonucleic acids, and quality-of-life parameters.
ConclusionsThe β-Tako trial will generate robust scientific evidence to address unmet clinical needs and inform clinical and treatment decisions in this uniquely challenging clinical entity.
The study has been registered (EU-CT number: 2023-510213-25-01, ClinicalTrials.gov Identifier, NCT06509074.
Keywords
Identify yourself
Not yet a subscriber to the journal?
Purchase access to the article
By purchasing the article, the PDF of the same can be downloaded
Price: 19,34 €
Phone for incidents
Monday to Friday from 9am to 6pm (GMT+1) except for the months of July and August, which will be from 9am to 3pm