T***he current control of low-density lipoprotein cholesterol among patients with atherosclerotic cardiovascular disease is very low and this is associated with an increase of cardiovascular outcomes. In addition, the latter this happens, the risk will be greater. This is mainly due to an insufficient use of the lipid-lowering therapy currently available. In fact, with current treatments (statins, ezetimibe and PCSK9 inhibitors), the majority of patients in secondary prevention should achieve low-density lipoprotein cholesterol goals. For these reasons, in this manuscript promoted by the Spanish Society of Cardiology we propose three simple and feasible decision-making algorithms that include the majority of clinical scenarios among patients with ischemic heart disease, with the double aim of attaining therapeutic goals in the majority of patients as soon as possible; in secondary prevention the magnitude of the benefit is risk- and time-dependent.
Keywords
Although major advances have been made in the diagnosis and treatment of atherosclerotic cardiovascular (CV) disease (ASCVD) in recent years, recurrent ischemic events are still very common.1 There are a number of reasons for this, including poor control of CV risk factors,2 among which hypercholesterolemia plays a major role.3 There is a wide body of evidence showing that excessive blood cholesterol is a major cause of ASCVD, in particular cholesterol that binds to low-density lipoproteins (LDL-C) and to apolipoprotein B-containing lipoproteins, which are represented by nonhigh-density lipoprotein cholesterol (non–HDL-C).4,5 The lowering thus of LDL-C and non–HDL-C using lipid-lowering drugs is a highly effective strategy for preventing both primary ASCVD and recurrent episodes.4,6 Clinical trials of drugs designed to reduce atherogenic cholesterol (statins,7 statins combined with ezetimibe,8 and PCSK9 inhibitors) have clearly shown that the greater the reduction in LDL-C levels, the greater the protective effect against ASCVD.9 Indeed, as yet, there is no limit to the amount that LDL can be lowered while still reducing CV risk, even when levels are below 30mg/dL.10,11 In addition, no harmful health effects have been observed in patients with very low LDL-C levels,12 although longer-term studies are needed. Data from numerous sources, including cases of rare genetic diseases characterized by very low LDL-C levels from birth, cellular and organ physiology studies, Mendelian randomization analyses, and clinical trials, have shown that very low LDL-C levels do not significantly alter physiological functions associated with cholesterol metabolism or increase the risk of disease.13
In view of the above evidence, CV prevention guidelines recommend increasingly strict atherogenic cholesterol targets for patients with ASCVD and tailoring these targets to different risk enhancers, such as ASCVD severity, comorbidities, and other CV risk factors.14 Guidelines also stress the importance of rapid attainment of targets to maximize the preventive benefit.15 In short, the goal for patients should be to reduce LDL-C levels “as much as possible and as fast as possible”. The European Society of Cardiology (ESC) recommends that patients with acute coronary syndrome (ACS) should be started on high-intensity statins as soon as possible, regardless of LDL-C levels.16
Despite the evidence, however, clinical practice studies continue to show that most patients with ASCVD do not meet the recommended treatment goals for the main CV risk factors. In the recent EUROASPIRE V registry, just one-third of coronary patients had LDL-C levels within the desired range.2,17 There are several reasons that could explain this lack of control, including frequent prescription of lipid-lowering therapies incapable of achieving sufficiently low LDL-C levels, a certain degree of physician inertia when it comes to intensifying therapy in patients not meeting their goals, and suboptimal treatment adherence among patients.18 Failure to achieve lipid goals is worrying as poor control is directly linked to high ASCVD recurrence rates and also has high social and economic costs.19,20 Strategies are thus needed to ensure that both clinicians and patients make appropriate use of available treatment resources.
The aim of this consensus statement is to help clinicians achieve LDL-C goals in patients with ASCVD in as short a time as possible. Special attention is placed on accurate assessment of CV risk, establishment of LDL-C goals, and identification of the most suitable lipid-lowering therapies for patients with acute or chronic coronary syndrome. This statement is not intended to be used as a clinical practice guideline, but rather to present practical recommendations that we believe will help improve LDL-C control in Spain. The statement is an initiative of the Spanish Cardiology Society and has been endorsed by the three sections of the society mostly closely involved in lipid control (Clinical Cardiology, Vascular Risk and Cardiac Rehabilitation, and Ischemic Heart Disease and Acute Cardiovascular Care).
ALGORITHMS: METHODOLOGY AND JUSTIFICATIONThe first step in the design of the algorithms presented in this study was for a group of hyperlipidemia experts from a range of clinical fields and settings (cardiology, primary care, lipid units) to examine evidence from different clinical trials. The purpose of the algorithms is to help physicians from any specialty (eg, cardiologists, internists, general practitioners) or care level (primary care, hospitals, outpatient clinics) achieve LDL-C goals in patients with ischemic heart disease in as short a time as possible. The algorithms were designed to address 2 major clinical scenarios: an acute scenario (hospitalization due to ACS or admission for myocardial infarction and/or coronary revascularization in the past year) and a chronic scenario (ACS >1 year previously, percutaneous or surgical coronary revascularization, or stable angina without revascularization).
The same 6-step procedure was applied to both scenarios to determine 1) individual CV risk, 2) prior use of statin therapy, 3) use of maximally tolerated statin doses, 4) LDL-C levels at initial evaluation, 5) treatment options for achieving LDL-C goals as soon as possible, and, 6), additional treatment options for achieving unmet goals after a period of 4 to 6 weeks for patients with acute disease or chronic disease and extreme risk or 4 to 8 weeks for patients with chronic disease and nonextreme (very high) risk.
In line with the 2017 and 2019 recommendations of the American Association of Clinical Endocrinologists, patients with ischemic heart disease were categorized into 2 CV risk groups—an extreme risk group and a nonextreme (very high) risk group—depending on their clinical characteristics.21,22 In light of recent evidence,8,11,23,24 however, it was decided to extend the criteria for extreme risk. While not all the patients in this group have the same risk of a CV event, they all have a higher risk than patients in the nonextreme (very high) risk group. Patients not meeting any of the criteria for extreme risk were considered to be at very high risk of a CV event (table 1).
Patients with ischemic heart disease and extreme or very high cardiovascular risk
| Extreme CV risk | • Diabetes• Chronic kidney disease (stage 3b A2-A3 or 4)• Atherosclerotic vascular disease in another place (clinical or clearly defined by imaging)• ≥ 2 previous myocardial infarctions• Myocardial infarction in previous 2 years• Involvement of ≥ 2 coronary vessels (multivessel disease), regardless of revascularization• Previous coronary revascularization surgery• ACS in young patient (<55 y for men and <65 y for women)• Progression of cardiovascular disease (clinical or confirmed by imaging) despite being within target range*• Familial hypercholesterolemia• Lipoprotein (a) >180 mg/dL |
| Nonextreme (very high) CV risk | • None of the above criteria |
ACS, acute coronary syndrome; CV, cardiovascular.
A target LDL-C level of under 55mg/dL was established for patients at extreme risk, regardless of the clinical scenario (acute or chronic). The choice of this lower threshold was based on the findings of several studies. The IMPROVE-IT trial, for example, found that intensive therapy with simvastatin 40mg plus ezetimibe 10mg (LDL-C, 53.7mg/dL) resulted in a significant reduction in CV events compared with simvastatin 40mg (LDL-C, 69.5mg/dL).8 In a meta-analysis of 8 clinical trials involving 38 153 patients allocated to statin therapy, those who achieved LDL-C levels under 50mg/dL experienced the fewest CV events.7 Recent studies of PCSK9 inhibitors have also shown that strict lipid goals can be achieved with these drugs. In the FOURIER trial (patients with ASCVD), evolocumab lowered median LDL-C levels to 30mg/dL,23 while in the ODYSSEY trial (patients with ACS in past year),11 alirocumab resulted in an initial lowering of LDL-C to 38mg/dL, although this increased to 53mg/dL at the end of follow-up due to the study design. In both studies, addition of PCSK9 inhibitors to standard treatment resulted in further reductions in CV events in patients with ischemic heart disease.11,23 For our algorithms, it was decided to maintain the LDL-C goal at under 70mg/dL for patients with chronic disease and nonextreme risk. Notwithstanding, if a patient experiences a new CV event at an LDL-C level of between 70 and 135mg/dL, the goal should be to reduce their LDL-C by at least 50%, especially if they have been previously treated with statins.25
We followed the recommendations of Masana et al.26–28 and the more recent recommendations of the Spanish Atherosclerosis Society,29 albeit with some minor modifications, to choose the most suitable lipid-lowering therapies and estimate their ability to reduce LDL-C (table 2).30 Although the bulk of evidence on the CV benefits of LDL-C reduction comes from clinical trials with statins (vs statins plus ezetimibe and PCSK9 inhibitors), priority was given to achieving target LDL-C levels as quickly as possible using existing lipid-lowering drugs with proven CV benefit.8,11,23
Classification of lipid-lowering drugs according to their ability to reduce low-density lipoprotein cholesterol
| Lowering ability | Treatments |
| Extreme (76-85% reduction) | Add PCSK9* inhibitor at maximally tolerated doses to lipid-lowering treatment• Evolocumab 140 mg (∼85%)• Alirocumab 75 mg (∼76%)• Alirocumab 150 mg |
| Very high (60%-75% reduction) | Potent statin + ezetimibe• Atorvastatin 40-80mg+ezetimibe 10mg• Rosuvastatin 10-40mg+ezetimibe 10mg |
| High (50%-59% reduction) | High-potency statin• Atorvastatin 40-80mg• Rosuvastatin 20-40mgMedium-potency statin + ezetimibe• Simvastatin 20-40mg+ezetimibe 10mg• Pravastatin 40mg+ezetimibe 10mg• Lovastatin 40mg+ezetimibe 10mg• Fluvastatin 80mg+ezetimibe 10mg• Pitavastatin 2-4mg+ezetimibe 10mg• Atorvastatin 10-20mg+ezetimibe 10mg• Rosuvastatin 5mg+ezetimibe 10mg |
| Moderate (30%-49% reduction) | Medium-potency statin• Atorvastatin 10-20mg• Rosuvastatin 5-10mg• Simvastatin 20-40mg• Pravastatin 40mg• Lovastatin 40mg• Pitavastatin 2-4mg• Fluvastatin XL 80mgLow-potency statin+ezetimibe• Simvastatin 10mg+ezetimibe 10mg• Pravastatin 20mg+ezetimibe 10mg• Lovastatin 20mg+ezetimibe 10mg• Fluvastatin 40mg+ezetimibe 10mg• Pitavastatin 1mg+ezetimibe 10mg |
Treatment adherence is an importance consideration when contemplating changes to treatment and patients must be made to understand the importance of adhering to treatment if they wish to achieve their goals. Finally, although decisions on whether to intensify lipid-lowering therapy in patients with ASCVD should not be delayed, patients should be encouraged to follow an appropriate diet and to exercise regularly.1,6,14,15,25
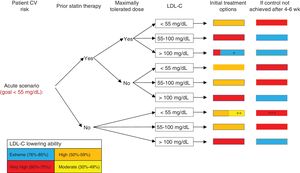
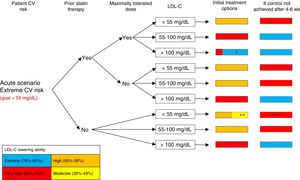
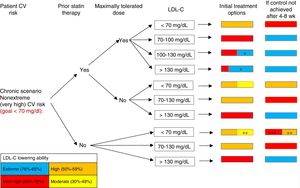
INTERPRETATION OF ALGORITHMSThis consensus statement proposes 3 algorithms for improving LDL-C control in different clinical scenarios in patients with ischemic heart disease and CV risk. Algorithm 1 is for patients with acute disease (figure 1), algorithm 2 is for patients with chronic disease and extreme risk (figure 2), and algorithm 3 is for patients with chronic disease and nonextreme (very high) risk (figure 3). The 3 algorithms are designed to be interpreted in a similar manner. Each algorithm has 6 columns. The first column identifies the patient's CV risk (extreme vs nonextreme/very high) and establishes the corresponding LDL-C goal. The second and third columns dictate which steps should be taken depending on whether the patient was taking statins at the time of evaluation and on whether they were receiving maximally tolerated doses. The fourth column shows a range of possible LDL-C levels during initial evaluation. Based on this information and on the LDL–C-lowering ability of the different treatments contemplated in table 2, the fifth column shows treatment intensification options for achieving target LDL-C levels in as short a time as possible. To simplify interpretation, this column uses the same color-code system as that used in table 2 (red, very high-intensity lipid-lowering treatment; orange, high-intensity lipid-lowering treatment; yellow, medium-intensity lipid-lowering treatment; blue, addition of PCSK9 inhibitors). A box containing 2 colors indicates that there are 2 potentially valid treatments. The size of each color indicates the approximate likelihood of reaching the desired LDL-C levels with each option. When an LDL-C reduction of 50% to 59% is needed, patients can be treated with maximally tolerated statin doses or moderate-intensity statins combined with ezetimibe, depending on the situation (eg, previous statin therapy and its intensity, risk of adverse effects with maximally tolerated statin therapy). Patients should have their blood tested after 4 to 6 or 8 weeks of treatment initiation, depending on the scenario. If target levels have not been reached, treatment should be intensified according to the indications in column 6, which also uses the same color codes as table 2. If, by contrast, target levels have been achieved, the follow-up visits should be spaced out.
Algorithm for lipid-lowering therapy in acute scenarios. CV, cardiovascular; LDL-C, low-density lipoprotein cholesterol. * Consider addition of PCSK9 inhibitors if combination statin and ezetimibe therapy is not expected to achieve goals (tailor to patient's LDL-C levels and characteristics). ** Consider moderate-intensity statins in elderly or frail patients or when high-intensity statins are contraindicated. *** If LDL-C reduction of ≥ 50% has not been achieved.
Algorithm for lipid-lowering therapy in chronic with extreme CV risk scenarios. CV, cardiovascular; LDL-C, low-density lipoprotein cholesterol. * Consider addition of PCSK9 inhibitors if combination statin and ezetimibe therapy is not expected to achieve goals (tailor to patient's LDL-C levels and characteristics). ** Consider moderate-intensity statins in elderly or frail patients or when high-intensity statins are contraindicated. *** If there is failure to reduce LDL-C by ≥ 50%.
Algorithm for lipid-lowering therapy in chronic with nonextreme (very high) CV risk scenarios. CV, cardiovascular; LDL-C, low-density lipoprotein cholesterol. * Consider addition of PCSK9 inhibitors if combination statin and ezetimibe therapy is not expected to achieve goals (tailor to patient's LDL-C levels and characteristics). ** Consider moderate-intensity statins in elderly or frail patients or when high-intensity statins are contraindicated. *** If there is failure to reduce LDL-C by ≥ 50%.
The REPAR study showed that good lipid control was achieved in only 26% of patients seen at cardiology clinics in Spain and that this lack of control was mainly due to the underutilization of available lipid-lowering treatments: only 45% of patients were being treated with high-intensity statins and 14% with ezetimibab.31 Physician inertia was also very common, as therapy was not intensified in 7 out of 10 patients with inadequate lipid control. These findings are surprising considering the strong evidence supporting the CV benefits of lipid-lowering therapy, whether with high-intensity statins, such as atorvastatin (which is specifically indicated for the prevention of CV disease), or with statins plus ezetimibe.32 Measures are needed to reverse this trend. The algorithms presented in this document are designed to provide a simple guide to improving lipid control for secondary prevention in Spain. They are informed by the latest scientific evidence and contemplate most clinical scenarios of ischemic heart disease, including hospitalization for ACS and outpatient monitoring.
Lowering of LDL-C levels reduces the incidence of CV events, and the lower the levels, the greater the reduction.12 These benefits are also seen in patients older than 75 years.33 Nonetheless, unlike other risk factors, such as hypertension and diabetes, LDL-C does not appear to be associated with a J-curve, ie, there is no threshold under which lowering of LDL-C starts to produce adverse effects.8,11,23 The LDL-C goal established for the extreme CV risk category, 55mg/dL, is particularly strict because evidence from various studies suggests that a threshold of 70mg/dL is too high for many patients with ischemic heart disease.8,11,23
CV risk tends to be underestimated across clinical practice settings.34 This is concerning, as risk underestimation in hyperlipidemic patients leads to inappropriate LDL-C goals and insufficient intensification of lipid-lowering treatment.25 The aim of this consensus statement was to formulate a simple approach for identifying ASCVD patients with extreme CV risk and to provide guidance on how to optimize lipid-lowering therapy and achieve LDL-C goals in this setting. A recent Spanish study described an equation for predicting risk in patients with familial hypercholesterolemia.35
The speed with which LDL-C goals are achieved is also important, as the sooner a target is reached and the longer it is maintained, the greater the benefit.5,36,37 The algorithms presented in this document are designed to ensure that patients reach target LDL-C levels as quickly as possible. If the treatment intensification initiated at the time of evaluation does not succeed in lowering levels within 4 to 6 or 8 weeks, depending on the scenario, further intensification is needed.
The current evidence indicates that the CV benefits of lipid-lowering therapy depend not so much on the treatment used, but on the LDL-C reduction achieved, thus shifting the focus from high-intensity statin therapy to high-intensity lipid-lowering therapy.38 The choice of lipid-lowering drugs should therefore be determined by each patient's LDL-C goals. Currently available lipid-lowering drugs with proven benefit for secondary CV prevention8,11,23 are shown in table 2, which also indicates how treatments can be combined depending on prior therapy and current goals. Again, the ultimate aim is to reach target LDL-C levels as quickly as possible.
A French research group recently published an algorithm with a similar purpose to ours. While they did not include the latest evidence on PCSK9 inhibitors or provide recommendations beyond 4 to 6 or 8 weeks following discharge for ACS,15 their algorithm led to improved lipid control through intensification of lipid-lowering therapy. At discharge, 91% of patients were taking high-intensity statins (combined with ezetimibe in 62%), and at follow-up, 77% of the patients had reached the target LDL-C level of under 70mg/dL.39 We believe it is also likely that the application of the algorithms proposed in this manuscript will lead to high rates of LDL-C control in patients with ischemic heart disease in different clinical scenarios and ultimately reduce the risk of new CV events. There is, however, a need for studies analyzing the cost-effectiveness of these algorithms in clinical practice.
ConclusionsAn optimal approach to secondary CV prevention should include lifestyle measures (with patient participation in cardiac rehabilitation programs), attainment of risk factor control goals, and use of drugs and interventions with proven prognostic benefits. Although it is well known that LDL-C lowering significantly reduces the risk of CV events, most patients do not reach their target levels, even though we have the tools to achieve adequate control in most cases. While this is worrying in the context of the general hyperlipidemic population, it is even more of a concern in patients requiring secondary prevention. To address this situation, the Spanish Cardiology Society decided to create a set of simple, easy-to-apply algorithms that cover most secondary prevention scenarios encountered in clinical practice.
FundingThis consensus statement was drawn up with an unconditional grant from MSD and Amgen. Neither of these companies was involved in any way or at any time in the preparation of this document. The opinions and recommendations of the experts are independent of the funding received.
Conflicts of InterestC. Escobar has received consultancy and/or speaker fees from MSD, Amgen, Mylan, Sanofi, Almirall, Recordati, and Esteve. M. Anguita has received consultancy and/or speaker fees from Amgen and MSD. V. Arrarte has received consultancy and/or speaker fees from Amgen, Sanofi, MSD, Ferrer, Servier, Pfizer, Esteve, and Rovi. V. Barrios has received consultancy and/or speaker fees from MSD, Amgen, Mylan, Sanofi, Almirall, Recordati, Rovi, Pfizer, and Esteve. Á. Cequier has received consultancy and/or speaker fees from Abbott Vascular, Biosensors, Amgen, Bayer, Biotronik, Boehringer Ingelheim, Ferrer International, Sanofi, and Medtronic. J. Cosín-Sales has received consultancy and/or speaker fees from MSD, Amgen, Ferrer, Mylan, Sanofi, and Esteve. E. López de Sa has received consultancy and/or speaker fees from Sanofi and MSD. L. Masana has received consultancy and/or speaker fees from Amgen, Sanofi, Daichii/Sankyo, Mylan, Servier, and MSD. V. Pallarés has received consultancy and/or speaker fees from MSD, Almirall, Servier, and Esteve. L. Pérez de Isla has received consultancy and/or speaker fees from Sanofi, MSD, and Amgen. X. Pintó has received consultancy and/or speaker fees from Mylan, Amgen, Sanofi, Esteve, Ferrer, Servier, and Rubió. J.R. González Juanatey has received consultancy and/or speaker fees from Amgen, Sanofi, and MSD. J.L. Zamorano has received grants and consultancy and/or speaker fees from Abbott and Edwards, Sanofi, and Philips. The other authors declare no conflicts of interest.