Keywords
INTRODUCTION
Detection of viable myocardium in patients with severe regional or global left ventricular dysfunction is important for determining the prognosis and therapeutic management of ischemic heart disease. The established techniques for assessing myocardial viability are low-dose dobutamine echocardiography and techniques using radioactive isotopes.1 Because of significant technological advances over the past years, cardiac magnetic resonance (CMR) imaging is being increasingly used for this purpose. With this technique, imaging of cardiac morphology as well as information on contractile function, perfusion, and the extent of myocardial necrosis are obtained in the same examination.1-4 Cardiac magnetic resonance offers various possibilities within the viability study, such as assessment of diastolic wall thickness, response to low-dose dobutamine, and preservation of flow in the area of infarction, as well as imaging with delayed contrast enhancement (DCE). The diagnostic accuracy of this last technique, which identifies the presence and extent of necrotic areas and determines myocardial viability, has been shown in several studies.1-5 Nevertheless, the relative role of each of these parameters for predicting improvement in dysfunctional myocardium following revascularization is uncertain.
The aim of this study was to determine the reliability of early analysis of the most extensively used CMR indicators for predicting recovery of systolic function and ventricular remodeling in an optimal scenario of patients with a first ST-segment elevation acute myocardial infarction (AMI), single vessel disease and patent culprit artery.
PATIENTS AND METHODS
Patients
The study included 17 patients with a first ST-segment elevation AMI, defined as typical chest pain of more than 30 minutes' duration, ST segment elevation >1 mm at 80 ms from the J point in more than one lead that did not normalize with nitroglycerin, and elevated markers of myocardial injury. The exclusion criteria were a history of known heart disease (ischemic or not), presence of an intercurrent disease that would make long-term follow up difficult, significant involvement of an artery that had not caused the infarction (>50% stenosis on coronary angiography), culprit artery not patent after pre-discharge catheterization, or contraindications for CMR (implants incompatible with the magnetic field). The ethics committee of our hospital approved the study and all patients gave informed consent to participate. Baseline characteristics of the study group are described in Table 1.
Initial CMR imaging was performed at 12±8 days after the infarction. Previously, all patients had undergone coronary angiography (7±5 days post-infarction) and, in cases of >50% residual stenosis (13 patients), angioplasty with or without stent placement. At 6 months CMR was repeated (180±30 days) to evaluate left ventricular changes, and repeat cardiac catheterization was done (180±12 days) to assess persistence of arterial patency (<50% stenosis), which was confirmed in all cases.
CMR Imaging Protocol
Cardiac magnetic resonance imaging was performed on a 1.5T system (Magnetom Sonata; Siemens, Erlangen, Germany) with a surface coil, prospective cardiac gating and breath-holding. Cine sequences for functional assessment (TrueFISP; TR: 25 ms; TE: 1.6 ms; flip angle: 61º; matrix: 256x128; slice thickness: 6 mm) were acquired in several planes (2-, 3-, and 4-chamber views and 1-cm short-axis views from the mitral valve to the apex) at rest and after intravenous administration of 10 μg/kg/min of dobutamine. Subsequently, at least 3 short-axis views were planned (basal, midventricular, and apical) for acquisition of first-pass myocardial perfusion sequences (TrueFISP; TI: 110ms; TR: 190 ms; TE: 1 ms; flip angle: 49º; matrix: 128x72) after administration of 0.1 mmol/kg of gadolinium (gadopentate dimeglumine; Magnograf®) at a rate of 3 mL/s. Sixty consecutive images were taken at each slice position. Ten minutes after contrast injection, inversion-recovery images (TrueFISP, TR: 700 ms; TE: 1.1; slice thickness: 6 mm, flip angle: 50º, matrix: 195x192, adapting inversion time in each case to "null" the myocardium signal) were acquired in views identical to the functional cine sequences in order to obtain DCE images.
The 6-month control CMR consisted in acquisition of functional cine images following the same protocol as in the initial examination.
No medication was interrupted for the CMR studies; 73% of patients were under treatment with beta-blockers and 8% with calcium channel blockers. Two examples of the CMR images obtained are shown in Figures 1 and 2.
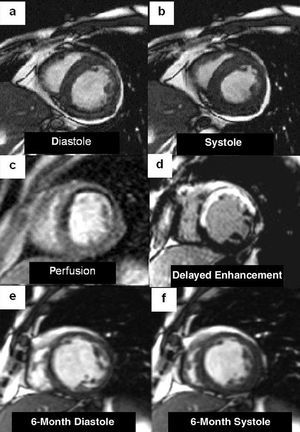
Fig. 1. Short-axis cardiac magnetic resonance images in a patient with anteroseptal infarction. Images from the initial cine study (a and b) show anterior and septal akinesia. First-pass myocardial perfusion clearly depicts hypointense areas in the anterior and septal aspects (c) and transmural delayed contrast enhancement affects the dysfunctional segments (d). The 6-month control images (e and f) show an absence of contractile improvement in the segments demonstrating hypoperfusion and delayed enhancement, as well as an increase in ventricular diameters.
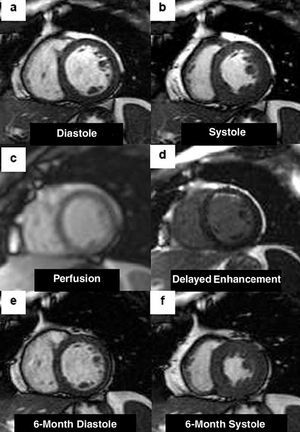
Fig. 2. Short-axis cardiac magnetic resonance images from a patient with anterior infarction. The initial cine images (a and b) depict anterior and anteroseptal wall akinesia. First-pass myocardial perfusion demonstrates homogeneous arrival of contrast to the entire myocardium (c). Delayed contrast enhancement is located in the anterior aspect and is <50% of wall thickness (d). The 6-month control images (e and f) clearly depict improved systolic function of the initially dysfunctional segments.
Analysis of CMR Images
The images acquired were analyzed using the Syngo platform with Numaris 4 software. The left ventricle was divided into 16 segments following the American Heart Association model.6 The ejection fraction (%) and ventricular volumes indexed to body surface area (mL/m²) were obtained from the short-axis cine sequences by Simpson's rule. Three short-axis cine sequences (basal, medial, and apical) were selected to measure diastolic wall thickness (DWT, in mm), baseline thickness (bT: systolic thickness-diastolic thickness, in mm) and thickening after low-dose dobutamine administration (dobT, in mm) in each segment. A segment was considered to have significant systolic dysfunction when the bT was <2 mm. Dobutamine response was considered positive when a dysfunctional segment presented a dobT of ≥2 mm.7
First-pass myocardial perfusion was assessed qualitatively and classified as normal (perfusion=1) or hypoperfusion (any delay or absence of contrast reaching the myocardium) (perfusion=0).
Transmural extent of delayed enhancement (thickness of enhanced area/total ventricular wall thickness x100%) was calculated from the images with myocardium suppression and classified into ranges (0%-25%, 26%-50%, 51%-75%, and 76%-100%).
In the 6-month CMR study we measured ventricular volumes, end-diastolic volume variation with respect to initial values (%), ejection fraction and wall thickening (6mT, in mm) following the same 16-segment model.
Definition of Myocardial Viability
An initially dysfunctional segment (bT<2 mm) was considered viable when it presented improved contractility at 6 months (6mT≥2 mm).
Statistical Analysis
All calculations were done with SPSS 9.0 (Chicago, Illinois, USA). Significance was set at a P-value of <.05. Continuous variables were expressed as mean ± standard deviation (SD). Categorical values were expressed as percentages of the study population and compared with the χ² test.
For the continuous variables (DWT and percentage of DCE), receiver operator characteristics (ROC) curves were analyzed to obtain the best cut-off for the detection of myocardial viability. Sensitivity and specificity were calculated for the four indicators studied, as well as positive and negative predictive values for the diagnosis of myocardial viability. Multiple logistic regression analysis, including the variables DWT, dobT, perfusion and DCE, was performed for the initially dysfunctional segments to determine the best predictor of myocardial viability.
For the analysis by patient, we obtained the mean value of each viability indicator in the segments belonging to the affected coronary territory (regional DWT, regional dobT, regional perfusion, regional DCE). The mean 6mT values for the affected coronary territories were also determined (regional 6mT). Bivariate correlations were obtained between the myocardial viability indicators (regional DWT, regional dobT, regional perfusion, regional DCE) and the 6-month ventricular parameters (end-diastolic volume index and end-systolic volume index, changes in end-diastolic volume, ejection fraction, regional 6mT), using the Pearson coefficient. Subsequently, linear multiple regression analysis was done for each 6-month ventricular parameter, including the indicators of cardiac viability that had shown a statistically significant correlation in each model.
RESULTS
Analysis by Segment
Among the 272 segments assessed at initial CMR imaging, 73 (27%) showed severe systolic dysfunction. Twenty-five (34%) had improved at the 6-month study. Using the values obtained for the initially dysfunctional segments, we calculated the diagnostic value of each indicator studied for predicting myocardial viability (Table 2).
Diastolic Thickness
The most effective cut-off point for predicting myocardial viability at 6 months was 5.5 mm (AUC=0.671 [0.545-0.797]; P=.014). There were 5 segments with lower diastolic thickness that did not improve at 6 months. Diastolic thickness ≥5.5 mm showed a sensitivity of 100%, specificity of 12%, positive predictive value of 47% and negative predictive value of 100% for the diagnosis of myocardial viability.
Inotropic Response
Among the 73 segments, 69 were analyzed with dobutamine (4 segments could not be assessed because of errors in image acquisition), and 15 (21%) of them showed a positive inotropic response. At the 6-month study, 80% of the segments showing a dobutamine response were viable, versus 32% of those that did not have an initial response (P=.001). Response to dobutamine had a sensitivity of 41%, specificity of 93%, positive predictive value of 80% and negative predictive value of 69% for predicting functional improvement.
Myocardial Perfusion
A total of 38 (52%) dysfunctional segments showed normal first-pass perfusion. Among them 66% improved at 6 months versus 20% of those that presented initial perfusion defects (P=.001). Normal perfusion had a sensitivity of 78%, specificity of 68%, positive predictive value of 66% and negative predictive value of 80% for diagnosing viability.
Delayed Contrast Enhancement
Delayed contrast enhancement was detected in 49 (67%) dysfunctional segments. Only 18% of segments with DCE improved, as compared to 96% of those without DCE (P<.0001). Absence of DCE had a sensitivity of 72%, specificity of 98%, positive predictive value of 96%, and negative predictive value of 82% as a predictor of myocardial viability.
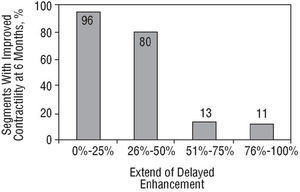
When DCE was classified into ranges, percentage of transmurality was <25% in 33%, 26%-50% in 7%, 51%-75% in 22%, and >75% in 38%. The number of segments that improved at six months was related with a smaller extent of DCE: improvement was 96% in those with <25% enhancement, 80% in those with 26%-50% enhancement, 13% in those with 51%-75% enhancement and 11% in those with >75% enhancement (Figure 3). The best cut-off point of extent of DCE for predicting functional improvement at 6 months was a transmurality of <50%, with a sensitivity of 81%, specificity of 95%, positive predictive value of 93% and negative predictive value of 87% (AUC=0.91 [0.83-0.99]; P<.0001).
Fig. 3. Percentage of segments with improved contractile function, based on extent of delayed contrast enhancement.
Multivariate Analysis
In the multivariate analysis of the four indicators studied, DCE transmurality was the only independent factor predicting myocardial viability at 6 months (odds ratio [OR]=84.5 [15.8-451.3]; P<.0001).
Analysis by Patient
In the analysis by patient, the 4 indicators of myocardial viability studied (regional DWT, regional dobT, regional perfusion, and regional DCE) were correlated with the parameters of regional systolic function (regional 6mT), global systolic function (ejection fraction) and 6-month ventricular volumes (Table 3). Regional DWT showed no significant correlations. Regional dobT correlated with end-systolic volume (r=-0.503; P=.0039), ejection fraction (r=0.584; P=0.014), and regional 6mT (r=0.572; P=.016). Regional perfusion correlated with end-diastolic volume (r=-0.505; P=.038), end-systolic volume (r=0.675; P=.0003), and ejection fraction (r=0.782; P<.0001). Regional DCE correlated with end-diastolic volume (r=0.609; P=.009), change in end-diastolic volume (r=0.509; P=.037), end-systolic volume (r=0.669; P=.003), ejection fraction (r=-0.725; P=.001), and regional 6mT (r=-0.827; P=.0001).
Multivariate Analysis
In the multivariate analysis of 6-month ventricular parameters, regional DCE was the only independent predictor of end-diastolic volume (R²=0.371; P=.009), change in end-diastolic volume (R²=0.259; P=.037), end-systolic volume (R²=0.447; P=.003), ejection fraction (R²=0.525; P=.001) and regional 6mT (R²=0.683; P=-.0001).
DISCUSSION
Among the CMR indicators of myocardial viability assessed in this study, the presence and quantitation of DCE was the most reliable for predicting improved contractility in segments with severe systolic dysfunction after a recent AMI. In addition, DCE was the best predictor of ventricular remodeling, and there was a correlation between the transmural extent of CDE and 6-month ventricular volumes and ejection fraction.
We found that a diastolic wall thickness of less than 5.5 mm ruled out the existence of viable myocardium. This result is in keeping with other studies affirming that wall thinning after infarction is a specific finding indicating an absence of myocardial viability or functional improvement.8-10 This parameter is limited, however, since it is an infrequent finding and has poor sensitivity.
Improved contractility following low-dose dobutamine seen in the present study was also a highly specific indicator of systolic functional recovery, but again, sensitivity was low. Some studies analyzing the inotropic reserve after infarction with CMR imaging have demonstrated high specificity and sensitivity for the detection of viability with this indicator.9-11 In contrast, Gunning et al12 found that the inotropic response to dobutamine showed high specificity (81%), but low sensitivity (50%) for the diagnosis of myocardial viability, as was our experience. In any case, the dose used and the fact that most of the patients were under treatment with beta-blockers or calcium channel blockers, which were not interrupted for the examination, may be the reason for the low sensitivity of the response to dobutamine in our study group.
We found that the presence of normal myocardial perfusion had acceptable sensitivity with somewhat lower specificity for predicting viability. Myocardial hypoperfusion in areas affected by the infarct and later revascularized represents the phenomenon of microvascular obstruction, in which blood flow is reduced even when the epicardial artery is opened.5,13 Several studies have shown that detection of hypoperfusion by CMR imaging is associated with an absence of viability5 and with a poorer prognosis following infarction,14 although the data on the relative value of this finding are contradictory. Finally, DCE was found to be the most reliable indicator for differentiating between necrotic and viable myocardium, and the extent of DCE was the best predictor of contractile improvement. The remaining indicators lost diagnostic importance in the analysis. Prior experimental15 and clinical5 studies have demonstrated that the use of DCE imaging allows characterization of tissues with high spatial definition and permits healthy myocardial tissue to be distinguished from necrotic tissue. The initial CMR studies based viability on the presence or absence of DCE. Kim et al16 designed the first study in which the transmural extent of DCE was analyzed, and showed that the percentage of segments in which contractile function improved decreased in parallel to the increase in transmurality. In later studies with a similar design, a similar inverse association was observed between DCE transmurality and systolic function following revascularization.17-19
One potential limitation to the use of DCE for defining myocardial viability is a possible overestimation of the size of the infarct. This potential pitfall has been described mainly in animal studies,20,21 and seems to be due to myocardial edema present in the acute/subacute phase following infarction, which contributes to increasing the size of DCE in the image.2 This phenomenon could partly explain the fact that 13% of segments with 51%-75% transmural DCE in our study and 11% of those with more than 75% DCE showed improvement at 6 months.
In the literature, the data on the relative value of perfusion and DCE for the diagnosis of myocardial viability are contradictory. Some studies contend that the presence of perfusion defects in patients with reperfused AMI indicates an absence of viability, regardless of the presence or not of DCE.22,23 Other studies, however, have shown that perfusion24-26 or dobutamine response26 do not provide additional information when the extent of DCE is assessed. Although detection of microvascular obstruction could be useful additional information since it has an adverse effect on healing, it should be remembered that hypoperfusion reflects only a part of the necrotic tissue affected by this phenomenon, not all the necrotic area, a fact that would limit the use of this factor alone to establish the diagnosis of viability. In any case, the differing results between studies could also be due to the use of different protocols for acquiring and analyzing the images. There is clearly a need for more extensive studies using standardized protocols to enable assessment of the relative value of each of these findings.
Quantification of DCE in the present study was reliable in both the analysis by segment and the analysis by patient, as evidenced by its relation with ventricular remodeling. In addition, DCE was the only independent predictor of 6-month ventricular volumes and ejection fraction, as well as variations in end-diastolic volume during follow-up. These results concur with findings reported by Choi et al,17 who showed an association between the percentage of DCE and contractile improvement in each segment, and the relationship of DCE with ventricular remodeling. Another similarly designed study found that early DCE quantification after AMI was the only independent predictor of ejection fraction at 6 months; other parameters such as dobutamine response and first-pass perfusion did not provide additional information.26 These data seem to indicate that viability is not a dichotomous entity, but instead that there are intermediate degrees between the complete absence of contractility and total normalization of contractility, such that the presence of a certain extent of revascularized viable myocardium could avoid posterior remodeling.
LIMITATIONS
One of the limitations of this study is the small number of participating patients; studies in larger series are required to confirm the findings. Another is the qualitative assessment used with first-pass perfusion. Quantitative evaluation of this variable might have increased the accuracy of the method.
CONCLUSIONS
Cardiac magnetic resonance imaging is a reliable diagnostic technique for the study of myocardial viability in patients with ischemic heart disease. This study analyzes the predictive power of several indicators of viability by CMR images obtained in an optimal scenario: early assessment in patients with a first reperfused AMI, patent artery, single-vessel lesion, and measurement of functional improvement at 6 months.
Among the parameters studied, DCE was the most accurate for detecting viable myocardium. Absence of DCE or less than 50% transmurality in segments with severe contractile alterations showed a high sensitivity and specificity for the diagnosis of viability. Moreover, this indicator was the best predictor of ventricular remodeling, thereby providing important prognostic information in patients who have experienced myocardial infarction.
See Editorial on Pages 803-805
Correspondence: Dra. M.P. López Lereu.
Unidad de Resonancia Magnética. Hospital Clínico Universitario.
Avda. Blasco Ibáñez, 17. 46010 Valencia. España.
E-mail: plereu@eresa.com