Cardiac contractility modulation therapy (CCMT) is a CE-marked device-based therapy for symptomatic patients with heart failure (HF) with a left ventricular ejection fraction <45%, poor quality of life, and frequent worsening heart failure (WHF) episodes despite guideline-directed medical therapy.1 CCMT is based on the release, by an implantable pacemaker-like device, Optimizer Smart (Impulse Dynamics Inc., USA), of high-voltage (≈ 7.5V) and long-duration (≈ 20 milliseconds) biphasic electrical signals on the septal wall of the right ventricle. These pulses are delivered during the absolute refractory period of the myocardium and consequently do not cause a new myocardial contraction but enhance cytosolic calcium regulation and, therefore, a positive inotropic effect. In addition to the positive inotropic effect, CCMT exerts several favorable actions that improve the overall biology of the failing myocardium.2 In addition, a levosimendan-like action (ie, calcium sensitization) has recently been reported.3
Because only a few parameters are currently known to be predictors of response to CCMT, this study aimed to evaluate whether the echocardiographic response to levosimendan could predict clinical and echocardiographic response to CCMT.
We prospectively and consecutively enrolled all patients undergoing elective Optimizer Smart implantation between October 2020 and October 2022. The patients’ demographic, clinical, and laboratory data were acquired 24hours before device implantation. The study was conducted according to the Declaration of Helsinki and was approved by the ethics committee of AORN dei Colli-Ospedale Monaldi (Resolution No. 903/2020). Signed informed consent was obtained from all patients.
As per the study protocol, levosimendan was administered in all patients intravenously as a continuous infusion at 0.2μg/kg/min up to a total dose of 12.5mg. The infusions were performed 72hours before the Optimizer Smart implant. No other intravenous drugs were administered to the patients during hospitalization.
The Optimizer Smart was implanted under local anesthesia. Two electrodes for delivering CCMT signals were placed on the right portion of the interventricular septum via the subclavian vein and successively connected to the implantable pulse generator. The Optimizer Smart was programmed to release therapy for 7hours daily in each patient.
In line with international recommendations,4 standard Doppler echocardiography and Doppler imaging were performed by nonblinded cardiologists after 48hours of levosimendan infusion and 6 months of CCMT. Following previous publications on cardiac resynchronization therapy, the echocardiographic response to levosimendan and CCMT was defined as ≥ 15% reduction in left ventricular end-systolic volume (LVESV).
WHF episodes, defined according to the latest recommendation of the Heart Failure Association of the European Society of Cardiology,5 were recorded at 1 year of follow-up, and the number of WHF episodes in the 12 months before implantation was used for comparison. Demographic and clinical variables are expressed as medians and standard deviations.
The Wilcoxon rank test was used to compare the differences between variables in cases of nonnormal distribution; a t test was used for variables with normal distribution. To assess the differences between the selected variables at enrollment and at 1 year of follow-up, we used repeated-measures analysis of variance. Because our study was self-controlled, no statistical adjustment methods for controlling confounding factors were used.
All statistical analyses were performed using Prism 10 (GraphPad Software, USA). All P values were 2-sided, and P <.05 was considered statistically significant. Most patients were male (n=12; 80%), and none had an ischemic etiology (n=8; 53%). The remaining demographic and clinical characteristics of the study population are summarized in table 1. No statistical differences were found in any clinical or echocardiographic variables between the 2 groups.
Clinical characteristics of the study population.
| Variables | Overall population (15) |
|---|---|
| Age, y | 57.9±8.7 |
| Female sex | 2 (13) |
| Ischemic etiology | 8 (53) |
| Hypertension | 7 (46) |
| Diabetes | 4 (26) |
| NYHA class II | 4 (26) |
| NYHA class III | 11 (74) |
| DR-ICD | 5 (33) |
| S-ICD | 2 (13) |
| CRT-D | 5 (33) |
| SBP, mmHg | 103±8 |
| DBP, mmHg | 74±3 |
| NT-proBNP, pg/mL | 1932±1238 |
| Atrial fibrillation | 5 (33) |
| LVEDV, mL | 203.2±65.2 |
| LVESV, mL | 122.3±39.5 |
| LVEF, % | 33.2±5.1 |
| LAVi, mL/m2 | 38.7±5.7 |
| Loop diuretic | 5 (33) |
| Beta-blockers | 11 (73) |
| ARNI | 12 (80) |
| MRA | 11 (73) |
| SGLT2i | 9 (60) |
ARNI, angiotensin receptor-neprilysin inhibitors; CRT-D, cardiac resynchronization therapy defibrillator; DBP, diastolic blood pressure; DR-ICD, dual-chamber implantable cardioverter-defibrillator; LAVi, left atrial volume index; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end-systolic volume; MRA, mineralocorticoid receptor antagonist; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; SBP, systolic blood pressure; SGLT2i, sodium-glucose cotransporter 2 inhibitors; S-ICD, subcutaneous implantable cardioverter-defibrillator.
Data are expressed as No. (%) or mean±standard deviation.
At 48hours, echocardiographic response to levosimendan occurred in 9 patients (60%) irrespective of the etiology (LEVO+), while there was no response in 6 patients (LEVO−). During follow-up, there were no changes in the doses of the disease-modifying drugs.
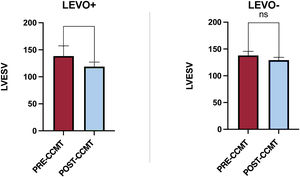
As shown in figure 1, after 6 months of CCMT, LVESV significantly decreased in the LEVO+ group (138.3±18.8mL vs 119.7±8.1mL; P <.05) compared with the LEVO− group (137.2±7.7mL vs 129.5±9.8mL; P=.48). At 1 year of follow-up, WHF episodes were significantly reduced post-CCMT in the LEVO+ group (14 vs 5; P=<.05).
Changes in left ventricular end-systolic volume after 6 months of CCMT in the LEVO+ and LEVO- groups. CCMT, cardiac contractility modulation therapy; LEVO+, patients with response to levosimendan; LEVO- patients without response to levosimendan; LVESV, left ventricular end-systolic volume; ns, not significant.
The main finding of our study is that echocardiographic response to levosimendan predicts clinical (ie, reduction in hospitalizations) and echocardiographic (reduction in LVESV) response to CCMT.
CCMT represents a new option for treating patients with HF, with a potential indication in approximately 1% to 5% of patients with HF with reduced ejection fraction/HF with mid-range ejection fraction.6 Levosimendan is an inodilator drug whose primary mechanism of action is sensitizing troponin C to intracellular calcium; a similar mechanism has recently been proposed for CCMT.
The observation that response to levosimendan predicts response to CCMT could be because these patients have greater contractile reserve and less myocardial fibrosis and are consequently more responsive to both therapies.
Because no clear criteria for predicting response to this new device-based therapy have been identified at this time, given the results of our study, the response to levosimendan could be helpful in decision-making on the Optimizer Smart implant.
This study has significant limitations, namely, its small sample size, its single-center nature, and its nonrandomized design. However, this study could be considered hypothesis-generating, and its results should be confirmed in future randomized clinical trials.
In conclusion, in this small single-center study, echocardiographic response to levosimendan infusion seemed to predict echocardiographic and clinical response to CCMT.
FUNDINGNone.
ETHICAL CONSIDERATIONSThe study was conducted according to the Declaration of Helsinki and was approved by the ethics committee of AORN dei Colli-Ospedale Monaldi (Resolution No. 903/2020). Signed informed consent was obtained from all patients. Possible sex/gender biases have been taken into account in the preparation of this article.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCENo artificial intelligence was used to prepare this article.
AUTHORS’ CONTRIBUTIONSD. Masarone collected and analyzed the data and wrote the first draft. The remaining authors contributed to the final revision of the first draft.
CONFLICTS OF INTERESTNone.