Heart retransplantation (ReHT) is controversial in the current era. The aim of this study was to describe and analyze the results of ReHT in Spain.
MethodsWe performed a retrospective cohort analysis from the Spanish Heart Transplant Registry from 1984 to 2018. Data were collected on donors, recipients, surgical procedure characteristics, immunosuppression, and survival. The main outcome was posttransplant all-cause mortality or need for ReHT. We studied differences in survival according to indication for ReHT, the time interval between transplants and era of ReHT.
ResultsA total of 7592 heart transplants (HT) and 173 (2.3%) ReHT were studied (median age, 52.0 and 55.0 years, respectively). Cardiac allograft vasculopathy was the most frequent indication for ReHT (42.2%) and 59 patients (80.8%) received ReHT >5 years after the initial transplant. Acute rejection and primary graft failure decreased as indications over the study period. Renal dysfunction, hypertension, need for mechanical ventilation or intra-aortic balloon pump and longer cold ischemia time were more frequent in ReHT. Median follow-up for ReHT was 5.8 years. ReHT had worse survival than HT (weighted HR, 1.43; 95%CI, 1.17-1.44; P<.001). The indication of acute rejection (HR, 2.49; 95%CI, 1.45-4.27; P<.001) was related to the worst outcome. ReHT beyond 5 years after initial HT portended similar results as primary HT (weighted HR, 1.14; 95%CI, 0.86-1.50; P<.001).
ConclusionsReHT was associated with higher mortality than HT, especially when indicated for acute rejection. ReHT beyond 5 years had a similar prognosis to primary HT.
Keywords
Heart retransplantation (ReHT) accounts for a very small proportion of all heart transplants (HT). In 2014, the International Society for Heart and Lung Transplantation published the characteristics and results of ReHT based on the experience of multiple centers in different countries, including some hospitals in Spain. According to the research, ReHT remained stable throughout the study period, with a frequency of 2% to 4%.1–5
Waiting lists have become increasingly long and may get longer gradually worldwide. Therefore, determining the factors related to this situation seems essential. Strong efforts have been made to increase the number of donors through donation after cardiac death programs or by accepting marginal donors, for example. However, a high proportion of transplants are performed in an acute setting due to the rise of mechanical circulatory support, which could have an impact on waiting lists. When to perform a transplant and in whom has not only become a major clinical decision but also an ethical dilemma. ReHT is a viable, and probably, the only therapeutic option for patients with severe graft dysfunction6,7 and identifying the ideal patient that could benefit from this procedure is essential.
The present analysis aimed to describe ReHT patient characteristics and survival in a nationwide transplant registry.8
METHODSThis is a retrospective cohort analysis based on data from the Spanish Heart Transplant Registry (SHTR), a multi-institutional, prospective database promoted by the Heart Failure Association of the Spanish Society of Cardiology from 1984 to December 2018. This database has been described elsewhere9 and comprises detailed clinical information on all HT procedures performed in Spain from 1984 to the present. The registry is updated yearly with data supplied by all Spanish transplant centers. The use of anonymized data for investigational purposes was approved by the local ethics committees of all the participating centers. For the purpose of this study, data from patients aged >16 years at the time of transplant regarding baseline recipient and donor characteristics, surgical procedure, immunosuppression, and survival were collected. We excluded patients with multiorgan transplants.
Baseline clinical characteristics and long-term post-HT survival of patients who underwent ReHT between 1987 and 2018 were compared with those of recipients of a first HT (no reHT group). For the former group, characteristics at the time of the second HT were used for analysis. Because urgent listing and use of circulatory support prior to HT were highly correlated, only the latter variable was used to avoid collinearity. Renal dysfunction was defined as a serum creatinine level >2mg/dL or the need for dialysis. Donor cytomegalovirus serostatus had a high rate of missing data and was not included in the analysis. The cause of death and the primary cause of ReHT were locally adjudicated by each participating center. The cause of ReHT was categorized as follows: primary graft failure (PGF) (that occurring primarily as a result of early allograft dysfunction in the absence of definite evidence of rejection), allograft rejection (AR) (both acute cellular and antibody-mediated rejection), cardiac allograft vasculopathy (CAV) (allograft dysfunction secondary to significant coronary disease according to the International Society for Heart and Lung Transplantation consensus statement10 or the presence of myocardial infarction by electrocardiographic or echocardiographic findings); and unspecific graft failure (UGF) (that without a definite diagnosis of acute rejection or CAV). Due to the similarities in key baseline characteristics between CAV and UGF as indications for ReHT (), we merged both groups for the present investigation. The timing of ReHT was categorized as early if occurring<1 year, midterm if 1-5 years, and late if >5 years from the previous transplant.
Primary endpointThe primary endpoint was posttransplant all-cause mortality or need for ReHT. ReHT reflects a second transplant in the ReHT group.
Statistical analysisContinuous variables are summarized as median (interquartile range [IQR]) and compared using the Mann-Whitney or Kruskal-Wallis test, as appropriate. Categorical variables are summarized as numbers and percentages and were compared using a chi-square test or the Fisher exact test, as appropriate.
Time to event (death or second HT) was modeled by means of parametric regression with Weibull distribution. To avoid the immortal bias derived from the fact that recipients of a retransplant had to be alive until the second HT, an additional time not available for the no-ReHT patients,11 we used marginal structural models in all analyses.12 Hence, in this study, the contribution of each patient was weighted by the inverse of its probability density function of having undergone ReHT conditioned on covariates of the study. Furthermore, to avoid the large variability in weights derived from the strong association between ReHT and some covariates (), we used stabilized weights13 defined as Probability(ReHT)/Probability (ReHT/covariates), where probabilities were estimated by logistic regression. In both numerator and denominator, the probability was P (ReHT) for patients with ReHT and 1 – P (ReHT) for patients with no ReHT.
The multivariable logistic regression model aimed to obtain the probability of ReHT was fitted with all single variables with significant association with the study outcome (). Previous sternotomy was a perfect predictor of ReHT and, consequently, it could not be entered in the model. For adjustment, this variable was entered in the parametric survival regression as an independent factor along with stabilized weight. Likewise, a second logistic regression model was fitted with exclusion of induction at the time of HT, and later used to calculate a different stabilized weight aimed at running a specific sensitivity analysis. Another sensitivity analysis was carried out excluding previous sternotomy from the survival regression analysis.
Missing data () were handled by multiple imputations using the fully conditional specification method, generating 10 imputed datasets using all applicable adjustment variables and the outcome variable as predictors. The average of the 10 imputed data sets carried out according to Rubin's rules14 was used for analysis. For imputation, categorical and continuous variables were modeled using logistic regression and linear regression, respectively.
All statistical tests were 2-sided and a P value <.05 was considered significant. Statistical analyses were performed using SPSS 25.0 (SPSS Inc, United States) and Stata 16.1 (StataCorp, United States).
RESULTSIn total, 7592 patients were identified in the SHTR database, of which 173 (2.3%) underwent ReHT at a median of 4.9 [IQR, 0.04-11.5] years after the initial transplant. The distribution of patients according to the indication for ReHT and time from initial transplant is outlined in figure 1A. Overall, CAV was the most frequent indication for ReHT (73 patients, 42.2%), at a median of 9.1 [IQR, 5.7-13.1] years after the initial transplant. Fifty-nine of the 73 patients (80.8%) were retransplanted more than 5 years after the initial transplant. AR led to ReHT in 16 patients (9.2%), at a median of 0.6 [IQR, 0.3-1.2] years, with most patients (75%) retransplanted within the first year after the initial transplant. UGF was the indication for ReHT in 43 patients (24.9%), occurring at a median of 9.0 [IQR, 1.7-12.8] years. Twenty-six (60.5%) and 11 (25.6%) of these patients were retransplanted more than 5 years and between 1 and 5 years after the initial transplant, respectively. PGF was the diagnosis in 41 patients (23.7%). All of them had been retransplanted within the first year post-HT. Additionally, both AR and PGF decreased as indications for ReHT over the observation period, whereas CAV and UGF increased (figure 1B).
Distribution of patients according to indication for retransplantation and time from the initial transplant (A) and indications for retransplantation by transplant era (B). AR, acute rejection; CAV, cardiac allograft vasculopathy; PGF, primary graft failure; UGF, unspecific graft failure.
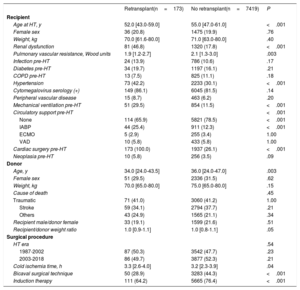
Patient demographics are outlined in table 1. Patients undergoing ReHT were younger (median 52.0 vs 55.0 years), and had an increased likelihood of pretransplant renal dysfunction (46.8% vs 17.8%), hypertension (42.2% vs 30.1%), mechanical ventilation (29.5% vs 11.5%), previous sternotomy (100.0% vs 26.1%), need for circulatory support with intra-aortic balloon counterpulsation (25.4% vs 12.3%), and longer cold ischemia time (median 3.3 vs 3.2hours). In contrast, retransplanted patients had lower pulmonary vascular resistance (median 1.9 vs 2.1 Wood units) and were less likely to undergo the bicaval technique (28.9% vs 44.3%). Donor age was also lower (median 34.0 vs 36.0 years).
Baseline characteristics of the population by to the study group
| Retransplant(n=173) | No retransplant(n=7419) | P | |
|---|---|---|---|
| Recipient | |||
| Age at HT, y | 52.0 [43.0-59.0] | 55.0 [47.0-61.0] | <.001 |
| Female sex | 36 (20.8) | 1475 (19.9) | .76 |
| Weight, kg | 70.0 [61.6-80.0] | 71.0 [63.0-80.0] | .40 |
| Renal dysfunction | 81 (46.8) | 1320 (17.8) | <.001 |
| Pulmonary vascular resistance, Wood units | 1.9 [1.2-2.7] | 2.1 [1.3-3.0] | .003 |
| Infection pre-HT | 24 (13.9) | 786 (10.6) | .17 |
| Diabetes pre-HT | 34 (19.7) | 1197 (16.1) | .21 |
| COPD pre-HT | 13 (7.5) | 825 (11.1) | .18 |
| Hypertension | 73 (42.2) | 2233 (30.1) | <.001 |
| Cytomegalovirus serology (+) | 149 (86.1) | 6045 (81.5) | .14 |
| Peripheral vascular disease | 15 (8.7) | 463 (6.2) | .20 |
| Mechanical ventilation pre-HT | 51 (29.5) | 854 (11.5) | <.001 |
| Circulatory support pre-HT | <.001 | ||
| None | 114 (65.9) | 5821 (78.5) | <.001 |
| IABP | 44 (25.4) | 911 (12.3) | <.001 |
| ECMO | 5 (2.9) | 255 (3.4) | 1.00 |
| VAD | 10 (5.8) | 433 (5.8) | 1.00 |
| Cardiac surgery pre-HT | 173 (100.0) | 1937 (26.1) | <.001 |
| Neoplasia pre-HT | 10 (5.8) | 256 (3.5) | .09 |
| Donor | |||
| Age, y | 34.0 [24.0-43.5] | 36.0 [24.0-47.0] | .003 |
| Female sex | 51 (29.5) | 2336 (31.5) | .62 |
| Weight, kg | 70.0 [65.0-80.0] | 75.0 [65.0-80.0] | .15 |
| Cause of death | .45 | ||
| Traumatic | 71 (41.0) | 3060 (41.2) | 1.00 |
| Stroke | 59 (34.1) | 2794 (37.7) | .21 |
| Others | 43 (24.9) | 1565 (21.1) | .34 |
| Recipient male/donor female | 33 (19.1) | 1599 (21.6) | .51 |
| Recipient/donor weight ratio | 1.0 [0.9-1.1] | 1.0 [0.8-1.1] | .05 |
| Surgical procedure | |||
| HT era | .54 | ||
| 1987-2002 | 87 (50.3) | 3542 (47.7) | .23 |
| 2003-2018 | 86 (49.7) | 3877 (52.3) | .21 |
| Cold ischemia time, h | 3.3 [2.6-4.0] | 3.2 [2.3-3.9] | .04 |
| Bicaval surgical technique | 50 (28.9) | 3283 (44.3) | <.001 |
| Induction therapy | 111 (64.2) | 5665 (76.4) | <.001 |
COPD, chronic obstructive pulmonary disease; ECMO, extracorporeal membrane oxygenator; HT, heart transplant; IABP, intra-aortic balloon pump; VAD, ventricular assist device.
Data are expressed as No. (%) or median [interquartile range].
Median follow-up was 5.8 [IQR, 0.8-12.8] years, with a significantly shorter follow-up in patients undergoing ReHT compared with primary HT (median, 2.0 years [IQR, 0.1-9.3] vs 5.9 years [IQR, 0.9-12.8]; P <.001).
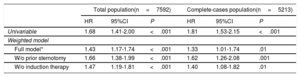
Retransplantation and outcomesIn total, there were 123 deaths (71.1%) and 4 second retransplants (2.3%) in the ReHT group compared with 4300 deaths (58.0%) in the no-ReHT group (P <.001). Patients undergoing ReHT were also more likely to die within 1 year (43.9% vs 22.9%, P <.001). On univariable analysis (), ReHT had worse survival than primary HT (unadjusted hazard ratio [HR], 1.81; 95% confidence interval [95%CI], 1.53-2.15; P <.001). In the weighted model, ReHT remained significantly associated with a worse outcome than recipients of a first, single HT (weighted HR, 1.43; 95%CI, 1.17-1.44; P <.001) (table 2).
Analysis to assess posttransplant mortality risk of the heart retransplantation compared with the no retransplantation population (marginal structural model)
| Total population(n=7592) | Complete-cases population(n=5213) | |||||
|---|---|---|---|---|---|---|
| HR | 95%CI | P | HR | 95%CI | P | |
| Univariable | 1.68 | 1.41-2.00 | <.001 | 1.81 | 1.53-2.15 | <.001 |
| Weighted model | ||||||
| Full model* | 1.43 | 1.17-1.74 | <.001 | 1.33 | 1.01-1.74 | .01 |
| W/o prior sternotomy | 1.66 | 1.38-1.99 | <.001 | 1.62 | 1.26-2.08 | .001 |
| W/o induction therapy | 1.47 | 1.19-1.81 | <.001 | 1.40 | 1.08-1.82 | .01 |
95%CI, 95% confidence interval; HR, hazard ratio.
Marginal structural model with inverse probability stabilized weighting and prior sternotomy. Inverse probability weighting included recipient characteristics (age at transplant, sex, weight, renal dysfunction, pulmonary vascular resistance, infection, diabetes, chronic obstructive pulmonary disease, hypertension, cytomegalovirus serology, peripheral vascular disease, mechanical ventilation, circulatory support, neoplasy), donor characteristics (age, sex, weight, cause of death, donor female/recipient male, recipient/donor weight ratio), surgical procedure characteristics (transplant era, cold ischemia time, surgical technique) and induction therapy at the time of transplantation.
We repeated marginal structural models after excluding both prior sternotomy and the use of induction therapy at the time of transplantation. On weighted analysis without prior sternotomy, ReHT was significantly associated with all-cause mortality/ReHT (HR, 1.66; 95%CI, 1.38-1.99; P <.001). Likewise, on weighted analysis without induction therapy at the time of transplantation, patients undergoing ReHT showed a worse prognosis (HR, 1.47; 95%CI, 1.19-1.81; P <.001) (table 2).
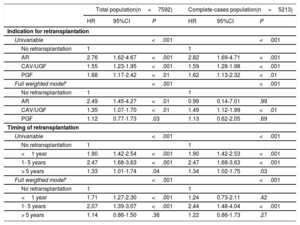
Effects of indication and timing of retransplantationOver the entire analysis, there were no significant differences in mortality/ReHT rates according to the indication for ReHT: 87.5% for AR, 72.6% for CAV, 73.2% for PGF, and 69.8% for UGD (P=.58). Similar findings were observed for mortality/ReHT within 1 year after transplant (56.3%, 35.6%, 51.2% and 46.5%, respectively; P=.26). Compared with the no-ReHT group, univariable regression showed a higher mortality risk for all the indications, particularly in patients undergoing ReHT for AR (unweighted HR, 2.76; 95%CI, 1.62-4.67; P <.001). In contrast, patients undergoing ReHT due to CAV/UGF showed the lower risk (unweighted HR, 1.55; 95%CI, 1.23-1.95; P <.001) (table 3). On weighted analysis, the effect on the risk of death/ReHT compared with the no-ReHT group was reduced but remained highly significant, particularly for AR and GVD/UGF (table 3).
Analyses to assess posttransplant mortality risk of the heart retransplantation compared with no retransplantation population (marginal structural model) by indication for retransplantation and timing of retransplantation
| Total population(n=7592) | Complete-cases population(n=5213) | |||||
|---|---|---|---|---|---|---|
| HR | 95%CI | P | HR | 95%CI | P | |
| Indication for retransplantation | ||||||
| Univariable | <.001 | <.001 | ||||
| No retransplantation | 1 | 1 | ||||
| AR | 2.76 | 1.62-4.67 | <.001 | 2.82 | 1.69-4.71 | <.001 |
| CAV/UGF | 1.55 | 1.23-1.95 | <.001 | 1.59 | 1.28-1.98 | <.001 |
| PGF | 1.68 | 1.17-2.42 | <.01 | 1.62 | 1.13-2.32 | <.01 |
| Full weighted model* | <.001 | <.001 | ||||
| No retransplantation | 1 | 1 | ||||
| AR | 2.49 | 1.45-4.27 | <.01 | 0.99 | 0.14-7.01 | .99 |
| CAV/UGF | 1.35 | 1.07-1.70 | <.01 | 1.49 | 1.12-1.99 | <.01 |
| PGF | 1.12 | 0.77-1.73 | .03 | 1.13 | 0.62-2.05 | .69 |
| Timing of retransplantation | ||||||
| Univariable | <.001 | <.001 | ||||
| No retransplantation | 1 | 1 | ||||
| <1 year | 1.90 | 1.42-2.54 | <.001 | 1.90 | 1.42-2.53 | <.001 |
| 1- 5 years | 2.47 | 1.68-3.63 | <.001 | 2.47 | 1.68-3.63 | <.001 |
| > 5 years | 1.33 | 1.01-1.74 | .04 | 1.34 | 1.02-1.75 | .03 |
| Full weigthed model* | <.001 | <.001 | ||||
| No retransplantation | 1 | 1 | ||||
| <1 year | 1.71 | 1.27-2.30 | <.001 | 1.24 | 0.73-2.11 | .42 |
| 1- 5 years | 2.07 | 1.39-3.07 | <.001 | 2.44 | 1.48-4.04 | <.001 |
| > 5 years | 1.14 | 0.86-1.50 | .36 | 1.22 | 0.86-1.73 | .27 |
95%CI, 95% confidence interval; AR, acute rejection; CAV, graft vascular disease; HR, hazard ratio; PGF, primary graft failure; UGF, unspecific graft failure.
Marginal structural model with inverse probability stabilized weighting and prior sternotomy. Inverse probability weighting included recipient characteristics (age at transplant, sex, weight, renal dysfunction, pulmonary vascular resistance, infection, diabetes, chronic obstructive pulmonary disease, hypertension, cytomegalovirus serology, peripheral vascular disease, mechanical ventilation, circulatory support, neoplasy), donor characteristics (age, sex, weight, cause of death, donor female/recipient male, recipient/donor weight ratio), surgical procedure characteristics (transplant era, cold ischemia duration, surgical technique) and induction therapy at the time of transplantation.
Regarding ReHT timing, death/ReHT rates were 78.3%, 96.3% and 62.8% for patients undergoing ReHT less than 1 year, between 1 and 5 years and more than 5 years after the initial transplantation, respectively (P=.002). Compared with the no-ReHT group, univariable regression showed a significantly higher risk for the 3 groups of different timings (table 3). On the weighted model, significant associations were found for those ReHT carried out less than 1 year after the first HT (HR, 1.71; 95%CI, 1.27-2.30) and between 1 and 5 years after the first HT (HR, 2.07; 95%CI, 1.39-3.07), P <.001 for both (table 3). However, ReHT performed more than 5 years after first HT showed no significantly different risk than no ReHT (table 3).
DISCUSSIONOverall, the results of the present study showed that ReHT was related to worse outcomes than primary transplantation. These results should be interpreted considering the characteristics of our study population. The ReHT rate in our country seems to be lower than that observed in other reports, particularly those from North America.15
In 2008, Atluri et al.16 described better ReHT survival compared with previous reports and mentioned careful perioperative management as one of the main pillars to explain this finding. Years later, an analysis of the United Network for Organ Sharing database4 reported that potential factors for longer survival were candidate selection, perioperative care, surgical technique, and advances in medical treatments. In this setting, the relationship between timing and indication for ReHT and outcome has been studied by multiple groups.1–4,17–27 Three publications3,25,26 between 2000 and 2005 reported that retransplants performed within 6 months after a HT were related to diminishing survival rates, significantly improving when timing and indications were taken into account. According to these studies, the optimal retransplant interval was over 2 years after the original HT and those with intractable AR in the first 6 months or PGF-related graft dysfunction needed to be excluded. These and previous experiences were summarized by The Working Group on Heart Retransplantation2 where chronic severe graft vasculopathy not medically nor surgically treatable, and chronic graft dysfunction with progressive heart failure in the absence of ongoing rejection were the only accepted indications for ReTH. Goerler et al.17 compared 30 day-mortality after retransplant. Their ReHT policy was reviewed years previously, considering acute graft failure as exclusion criteria for ReHT. Early ReHT was defined as retransplant within the first month post-HT and included patients with AR, right heart failure, biventricular failure, or acute graft failure of unknown origin. The 30-day mortality in this group was 3 times higher than that for the late retransplant group. We obtained similar findings in our series. According to our results, retrasplants performed between 1 and 5 years posttransplant were associated with the highest mortality. Nonetheless, those ReHT performed> 5 years after the first HT were not related to poorer outcomes. These factors could be explained by several factors, but probably the most important are the indications for ReHT and the transplant era. CAV and UGD accounted for more than 60% of all retransplants, with most of them being performed beyond 5 years from HT. In contrast, PGF and AR were more frequent in the first era of our study, all PGF retransplants and more than half of AR occurring within the first year post-HT. This change of trend appears to be in line with the progressive clinical awareness of the poor results with AR and the high mortality of ReHT patients in their first year compared with HT seen by other groups. For these patients, the use of short-term circulatory support devices seems a reasonable approach to achieve hemodynamic stability. In our analysis, we have found both AR and CAV to impact prognosis, the former being related to a very high risk of mortality. The exact explanation for CAV as a risk factor may need further investigation as several other factors could be involved, such as the transplant era, donor-related factors, etc.
It cannot be denied that a comprehensive selection of the best candidates is also essential. Hitherto, several factors have been implicated as risk factors for poor outcomes in retransplantation3,4,18,21,22,28 the most frequent ones being the recipient's age, and the need for extracorporeal membrane oxygenation or ventilation. For Kilic et al.28 among all the potential risk factors, the significant factors were age, the need for ventilation and chronic kidney disease. The presence of these 3 factors increased the risk of graft dysfunction to 32% at 5 years. In our sensitivity analysis, the exclusion of prior sternotomy increased the strength of the association between ReHT and mortality but the use of antibody induction therapy on weighted analysis maintained the strength of this association. These findings seem to support the notion that previous surgery could drive, at least partially, the worse prognosis of ReHT. For Miller et al.,15 among the several factors that could impact survival after ReHT, prior sternotomy was one of the most important factors related to outcomes after late retransplantation.
Nevertheless, the role of pretransplant immunologic status is of interest and needs further investigation, as has already been discussed in the literature.15 Moreover, there are no specific recommendations regarding the immunosuppression regime in ReHT, an issue already noted by Johnson et al.2 in 2007. In our series, induction therapy is less frequently used in ReHT than in HT. There is a need for individualized immunosuppression therapy after balancing risk-benefits in retransplantation, as patients are already immunosuppressed. It seems reasonable to reserve induction therapy for those with a high risk for early graft failure (patients who are more sensitized, depending on ReHT indication, recipient age, etc.) without prior immunosuppression-related significant adverse effects. In addition, short exposure to the adverse effects of prolonged immunosuppression could explain the better results observed in patients undergoing retransplant within the first year after the initial transplant. When balancing the risk-benefit of immunosuppression, malignancies are a major concern. ReHT patients had more comorbidities than HT patients, including a higher proportion of tumor development probably related to immunosuppressive therapy exposure.
Our study has the inherent limitations of all observational retrospective studies. Even the use of multivariable methods could not totally eliminate the possibility that occult biases persist. One of the most frequent and subtle biases usually present in this kind of study is the time or immortal bias. There are several methods, such as marginal structural models used in our study, to deal with this bias, which should be mandatory in this type of study. The small sample size derived from the analysis of population subsets could decrease the statistical power of some analyses. The absence of reliable information regarding allosensitization status is a major limitation of our study because patients undergoing retransplantation are more highly sensitized and a high sensitization portends increased posttransplant risk. Indeed, we found that the use of antibody induction therapy conferred retransplant recipients with a similar posttransplant risk as patients with a primary transplant. To adjust the possible lack of data, we used multiple imputation. Multiple imputation is a well-recognized technique in dealing with missing data, typical of registry-based datasets. In our study, the results derived from the imputed dataset and complete-data population did not reveal relevant inconsistencies beyond those attributable to the smaller sample size of the latter. Furthermore, this is the first morbidity and mortality study that analyzes the situation of ReHT in Spain, with a very large number of cases, comparable to international experiences.
CONCLUSIONSOur results confirm that retransplant is associated with worse survival. AR and an intertransplant interval of 1 to 5 years seems to portend a particularly high risk of poor results. Overall, these findings could help to properly allocate allografts in an era of shortage of donors.
- -
Heart retransplantation represents a low proportion of all heart transplants.
- -
Survival of retransplant patients has been repeatedly reported to be low in relevant literature.
- -
This is the first report of the characteristics and results of heart retransplantion in Spain.
- -
Although patients undergoing retrasplantation were younger, they had a higher incidence of renal disease, the need for ventricular support or mechanical ventilation, and longer cold ischemia time.
- -
Mortality was higher in the retransplant group, with acute rejection being particularly related to poor outcome.
- -
Retransplantation 5 years after the first transplant has a similar prognosis to primary heart transplant.
None.
AUTHORS’ CONTRIBUTIONSAll authors contributed equally to the design, writing and development of this research.
CONFLICTS OF INTERESTNone to declare in the scope of this article.
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.rec.2021.06.009