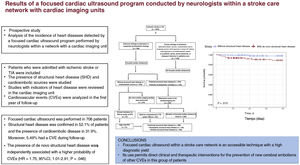
Recently, neurologists have begun to perform focused cardiac ultrasound for the detection of a cardiac source of embolism in stroke patients, requiring them to undergo a prior accredited training process. We designed a prospective study to analyze the incidence of heart disease detected by a focused cardiac ultrasound program within a stroke care network with cardiac imaging units and to identify the outcomes of detected structural heart disease at 1 year of follow-up.
MethodsWe included patients admitted to a university hospital for ischemic stroke or a transient ischemic attack between 2017 and 2021 who were evaluated by focused cardiac ultrasound. We studied the presence of structural heart disease and cardioembolic sources. We analyzed cardiovascular events (CVE) during the first year of follow-up.
ResultsFocused cardiac ultrasound was performed in 706 patients. Structural heart disease was detected in 52.1% and a cardioembolic source in 31.9%. Adverse CVE occurred in 5.49% of the patients in the first year of follow-up. The presence of de novo structural heart disease was independently associated with a higher probability of adverse CVE (HR, 1.72; 95%CI, 1.01- 2.91; P=.046).
ConclusionsFocused cardiac ultrasound within a stroke care network with cardiac imaging units is an accessible technique with high diagnostic yield. Its use allows clinical and therapeutic actions in the prevention of stroke recurrences and other CVEs in this group of patients.
Keywords
Ischemic stroke is a condition with high incidence that causes considerable disability: it is the leading cause of dependence in adults and the second most common cause of dementia worldwide. Its global incidence is notably increasing due to life expectancy improvements.1 In Spain, it is the second most common cause of death overall and the leading cause of death in women.2 Up to 25% to 30% of ischemic strokes are determined to have an underlying heart disease causing the brain embolism.3–5 The most frequent such condition is atrial fibrillation (AF), although other structural defects are also associated with high cardioembolic risk, such as depressed ventricular function, akinetic areas, and the presence of vegetation, intracavitary thrombi, and cardiac tumors. Other diseases have also been identified as potential causes of cardioembolic stroke but with lower risk, including patent foramen ovale (PFO), left atrial dilatation, and aortic atheromas.6 For this reason, the latest AHA/ASA guidelines, published in 2021, recommend the systematic use of transthoracic echocardiography (TTE) for the secondary prevention of ischemic stroke in patients with ischemic stroke of undetermined or cryptogenic source.7
However, TTE is not always immediately available due to health care overload in cardiac imaging units, which can prolong the length of stay and delay the confirmatory diagnosis. With recent technological developments and advances in portable ultrasound equipment, echocardiography has become accessible to other noncardiological professionals, such as primary care physicians, internists, intensivists, and neurologists, and the term “focused cardiac ultrasound” has arisen to refer to the use of ultrasound imaging by the physician attending the patient as an adjunct to the conventional physical cardiac examination. Focused cardiac ultrasound is used to answer specific questions to provide a diagnostic perspective on a relevant condition, is indicated in specific clinical situations, defined by patients’ symptoms and the clinical setting, and has a limited, nonexhaustive, character that stresses a high negative predictive value.8
In 2019, the Spanish Society of Cardiology (SEC) created a training and accreditation program on focused cardiac ultrasound for neurologists and other specialists.9
The systematic implementation of focused cardiac ultrasound performed by neurologists within the health care process of stroke units and in coordination with cardiac imaging units is not widespread and its usefulness has not been evaluated. Accordingly, we designed a prospective study of patients with ischemic stroke or transient ischemic attack (TIA) assessed using focused cardiac ultrasound within a network with cardiac imaging units to determine the incidence and type of structural disease detected and if it is associated with a higher incidence of cardiovascular events (CVEs) at 1 year of follow-up.
METHODSA prospective observational study was performed of consecutive patients admitted for ischemic stroke who were assessed using focused cardiac ultrasound by neurologists in the stroke unit of the neurology department of a university hospital from 2017 to 2021.
In 2019, a Focused Cardiac Ultrasound certification was developed by the Cardiac Imaging Section of the SEC and the Spanish Society of Neurology.9 The present study involved the participation of a neurologist with experience with cerebrovascular pathology and echocardiography, with 6 months of training in a cardiac imaging unit, and with accreditation in focused cardiac ultrasound.
From 2017 to 2021, studies were performed using the Toshiba Xario-200 portable ultrasound system with spectral Doppler and color Doppler capabilities and the ability to obtain indexed measurements of cardiac cavities. This system permits a complete heart evaluation and subsequent digitization and transmission via a network to the medical image repository of the cardiac imaging unit (EchoPAC Software, GE Healthcare, Spain) of the center, for image review and storage.
The criterion for focused cardiac ultrasound was hospitalization for an ischemic stroke or TIA of unknown etiology at the time of admission or of suspected cardioembolic etiology. Patients with a known stroke etiology at admission were excluded: atherothrombotic stroke due to severe carotid atheromatosis or unilateral occlusion at the ischemic lesion, lacunar stroke and cardioembolic stroke due to known moderate-to-severe mitral stenosis, or previously known AF without anticoagulants or at subtherapeutic doses (paroxysmal or permanent).10 Also excluded were patients with mechanical or biological heart valve prostheses because a comprehensive echocardiogram is already indicated in these patients, cases of severe dementia and high dependency due to stroke, defined as a modified Rankin score ≥ 5,11 and patients with malignant cerebral infarction receiving palliative care.12
The first 100 focused cardiac ultrasound studies were reviewed by cardiologists from the imaging unit, and high concordance was obtained between the 2 specialists for the screening of a cardioembolic source and structural heart disease (SHD). Subsequently, all studies in which the neurologist detected a potential SHD or cardioembolic source were reviewed in the cardiac imaging unit.
The final diagnosis of SHD was determined by the presence of any of the features listed in table 1.13–16
Criteria for the final diagnosis of structural heart disease13–16
| Moderate-to-severe LVH | In women: IVS ≥ 13 mmIn men: IVS ≥ 14 mm |
| Atrial septal aneurysm | Maximum excursion of the interatrial septum > 10mm or a total combined left-right excursion ≥ 15 mm |
| PFO with high embolic risk | PFO with right-left shunt: onset of spontaneous flow or passage of microbubbles toward the LA in the first 3 beats after contrast filling of the right atriumLarge PFO (≥ 2 mm)Presence of other characteristics:• ASA and hypermobile septum• Moderate-to-severe shunt (≥ 30 bubbles/3 beats)• Prominent Eustachian valve• Chiari network |
| Regional changes in left ventricular contractility | Akinesia or dyskinesia of more than 1 segment |
| Dilated LA | Indexed volume of the LA > 41 mL/m2 |
| Significant left valvular heart disease | Moderate or severe aortic regurgitation or stenosisModerate or severe mitral regurgitation or stenosis |
| Reduced LVEF | Women: LVEF < 54% (measured by Simpson)Men: LVEF < 53% (measured by Simpson) |
| Infectious endocarditis of the native valve | Presence of vegetationCardiac abscess or fistula |
| Intracavitary thrombus | AtrialVentricular |
| Cardiac tumor | Papillary fibroelastomaMyxoma |
| Aortic root dilatation | In any of the following anatomical regions:• Aortic ring: ≥ 13±1 mm/m2• Valsalva sinuses: ≥ 19±1 mm/m2• Sinotubular junction: ≥ 15±1 mm/m2• Tubular ascending aorta: ≥ 15±1 mm/m2 |
ASA, atrial septal aneurysm; IVS, interventricular septum; LA, left atrium; LV, left ventricle; LVEF, left ventricular ejection fraction; LVH, left ventricular hypertrophy; PFO, patent foramen ovale; SD, standard deviation.
SHDs were considered to be cardioembolic diseases if they necessitated a change to the clinical and therapeutic management for the secondary prevention of new cerebral embolisms: a) PFO with high embolic risk; b) regional changes in left ventricular contractility; c) left atrium with moderate-to-severe dilatation; d) infectious endocarditis; e) intracavitary thrombus; f) left ventricular dysfunction with a left ventricular ejection fraction < 35%; g) cardiac tumor; and h) left valvular heart disease in patients with moderate-to-severe mitral or aortic stenosis.13–15,17–23
Up to 2 types of SHDs and cardioembolic sources were recorded in each patient.
Comprehensive TTE was indicated in the following situations: a) study of significant left valvular heart disease; b) imaging results indicative of intracavitary thrombus; c) evaluation of regional contractility changes incorrectly evaluated by the focused cardiac ultrasound based on the criteria of the imaging unit cardiologist; and d) images of potentially cardioembolic sources suggesting vegetations or cardiac tumor masses.
Transesophageal echocardiography (TEE) was indicated in the following situations: a) study of PFO in patients younger than 60 years with stroke of undetermined source, and b) images of potentially cardioembolic sources indicating vegetations, cardiac tumor masses, or suspected atrial thrombus.16,24,25
In the transesophageal study of PFO, contrast enhancement was performed with a 10-mL shaken solution of a volume expander with gelatin succinate (Gelaspan).24
We used 24-hour Holter-ECG monitoring in all patients with undetermined stroke and 30-day monitoring in patients with stroke of undetermined source with high suspicion of cardioembolism to detect paroxysmal AF, as in patients with moderate or severe left atrial dilatation15,26 detected by focused cardiac ultrasound and reviewed in the cardiac imaging unit.
When the etiological study was completed, the etiology of the ischemic stroke and TIA was classified using the TOAST criteria3 as cardioembolic, atherothrombotic, lacunar, undetermined, and stroke of other determined cause. The National Institutes of Health Stroke Scale (NIHSS) was used to numerically score stroke severity: minor, < 4; moderate, < 16; severe, < 25; and very severe, ≥ 25 points.27,28 At 3 months, the modified Rankin Scale was used to assess poststroke functional capacity, using the following scoring system: 0, no symptoms; 1, no significant disability; 2, slight disability; 3, moderate disability; 4, moderately severe disability; 5, severe disability; and 6, dead.11
Focused cardiac ultrasound was used to analyze the cardiac rhythm of the patients assessed: sinus rhythm, AF, and pacemaker rhythm. An abnormal heart rhythm was not by itself considered a SHD.
After all of the information provided by the imaging studies was received, the following measures were taken for clinical and therapeutic management: a) if PFO with high embolic risk was confirmed, percutaneous occlusion was indicated in patients younger than 60 years and with ischemic stroke or TIA after the exclusion of other causes via a complete etiological study24; b) if moderate or severe left atrial dilatation in sinus rhythm was found, 30-day Holter-ECG was indicated to detect paroxysmal AF15,16; c) if infectious endocarditis was found, antibiotics were indicated, as well as surgery in selected cases29; d) if there were findings of intracavitary thrombus, anticoagulant therapy with unfractionated heparin sodium was started until thrombus resolution; e) in the case of left ventricular dysfunction with LVEF < 35%, regional changes in contractility with akinesis or dyskinesia, or moderate-to-severe mitral stenosis, anticoagulation with vitamin K antagonists was indicated in secondary prevention23; and f) if there was a cardiac tumor, surgical resection was indicated.30
The following CVEs were evaluated after discharge during the first year of follow-up: a) new ischemic stroke or TIA; b) acute myocardial infarction; c) emergency department attendance due to heart failure event with or without the need for hospital admission; and d) diagnosis of de novo AF.
To analyze the differences between the CVEs identified during the follow-up of patients based on whether they had SHD or not, we considered patients with a diagnosis of de novo SHD.
The research team adhered to Good Clinical Practice and Declaration of Helsinki guidelines and to those governing data protection and the handling of medical records. The study was approved by the Ethics Committee of University Clinical Hospital of Santiago de Compostela with the registration code 2022/162.
Because the study used health care data, we ensured adherence to the rules established in Additional Provision 17.a of Organic Law 3/2018, of December 5, concerning the protection of personal data and guarantee of digital rights. The procedure was followed for the use of pseudonymized data in research. Given that patients’ express consent was not obtained, we guaranteed a technical and functional separation between the research team and those who performed the data pseudonymization and storage.
Data were collected from the health care registries BICHUS and UNIDAD DE ICTUS (REDCAP), which gathered the clinical information of patients treated in the stroke unit of the center, and the information was completed with the data obtained from the focused cardiac ultrasound, TEE, and TTE studies.
Statistical analysis was performed with SPSS version 28 software (SPSS Inc, United States) and with R version 4.1.0. The kappa statistic was evaluated to measure the concordance of the findings obtained for the first 100 focused cardiac ultrasound studies performed by neurologists and those obtained using comprehensive echocardiography by cardiology imaging specialists. In this context, the probability of a random agreement was 0.5.
For continuous variables, we used mean±standard deviation, 25th and 75th percentiles, and minimum and maximum values after the removal of outliers. Differences were tested between patients with and without SHD using a Wilcoxon test for continuous variables. For categorical variables, absolute and relative frequencies were used. Differences between 2 groups were evaluated using the Fisher test. A Cox regression analysis was used to analyze the variables that were independently associated with CVE development. Hazard ratios (HRs) were adjusted by variables with P < .4 in the bivariate analysis. Results are expressed as adjusted HRs with their 95% confidence intervals (95%CIs). A Kaplan-Meier model was used to calculate the disease-free survival until the occurrence of a CVE before the end of follow-up. All P values < .05 were considered significant in all tests.
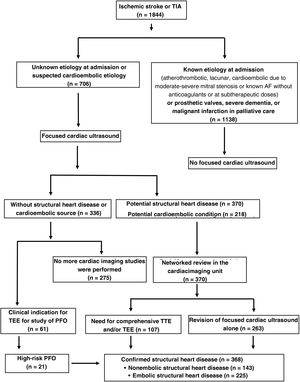
RESULTSBetween 2017 and 2021, 1844 patients were admitted with ischemic stroke or TIA and 38.3% (n = 706) underwent focused cardiac ultrasound; 33 had had a TIA and 673 an ischemic stroke. We excluded 61.7% (n = 1138) for meeting at least 1 exclusion criterion.
A concordance test was applied to the first 100 focused cardiac ultrasound studies performed for SHD or cardioembolic source screening. The concordance between the 2 groups was 0.98. The kappa coefficient was (0.98-0.5) / (1-0.5) = 0.48 × 2 = 0.96.
Of the 706 patients assessed using focused cardiac ultrasound, a potential SHD or potential cardioembolic source was detected in 52.4% (n = 370) and 30.8% (n = 218), respectively. In the patients without detected heart disease but with a clinical indication for the study of PFO, TEE was indicated (n = 61).
Studies with findings of heart disease were reviewed in the cardiac imaging unit. In 263 patients, only the focused cardiac ultrasound was reviewed because it was considered conclusive; the remainder was indicated comprehensive TTE or TEE. After the revision of the focused cardiac ultrasound, comprehensive TTE, and TEE, SHD was confirmed in 52.1% (n = 368); of these, embolic SHD was detected in 31.9% (n = 225) and nonembolic SHD in 20.3% (n = 143) (figure 1).
Schematic representation of inclusion and exclusion criteria and of the workflow regarding the strategy of focused cardiac ultrasound integrated within a stroke unit network with a cardiac imaging unit in patients admitted for ischemic stroke or TIA. AF, atrial fibrillation; TEE, transesophageal echocardiography; TIA, transient ischemic attack; TTE, transthoracic echocardiography.
TEE was performed in 120 patients (16.9%) and comprehensive TTE in 68 (9.6%); 20 patients required both studies.
Taking into account that up to 2 types of heart diseases were detected in each patient, table 2 lists the number and type of heart diseases detected by focused cardiac ultrasound, those confirmed after assessment in the imaging unit, and those evaluated by comprehensive TTE or TEE.
Types of heart diseases detected in focused cardiac ultrasound and types of heart diseases confirmed after assessment in the cardiac imaging unit. Frequencies of heart diseases of each category and those evaluated by TEE and comprehensive TTE
| Potential findings in focused cardiac ultrasound | Assessment in cardiac imaging unit | ||||
|---|---|---|---|---|---|
| Type of heart disease detected | N | Comprehensive TTE assessment | TEE assessment | Type of heart disease confirmed | N |
| Moderate-to-severe LVH | 152 | 5 | 1 | Moderate-to-severe LVH | 152 |
| Moderate or severe dilated LA | 85 | 10 | 3 | Moderate or severe dilated LA | 85 |
| Regional changes in contractility | 63 | 13 | 1 | Regional changes in contractility | 63 |
| Atrial septal aneurysm | 42 | 1 | 30 | PFO with high embolic risk with ASA | 19 |
| Significant aortic stenosis | 12 | 12 | 0 | Moderate-to-severe aortic stenosis | 12 |
| Significant aortic regurgitation | 12 | 8 | 1 | Moderate-to-severe aortic regurgitation | 8 |
| Significant mitral stenosis | 5 | 5 | 2 | Moderate-to-severe mitral stenosis | 5 |
| Significant mitral regurgitation | 10 | 10 | 3 | Moderate-to-severe mitral regurgitation | 10 |
| Potentially embolic valve image awaiting characterization | 21 | 11 | 18 | Infectious endocarditis | 9 |
| Fibroelastoma | 2 | ||||
| Calcium nodules, Lambl excrescences, or artifacts | 10 | ||||
| Reduced LVEF | 29 | 10 | 0 | Reduced LVEF | 29 |
| Image indicating intracavitary thrombus | 14 | 7 | 3 | Intracavitary thrombus | 9 |
| Artifacts | 5 | ||||
| Aortic root dilatation | 11 | 3 | 0 | Aortic root dilatation | 11 |
| Dilated cardiomyopathy with LVEF < 35% | 10 | 3 | 0 | Dilated cardiomyopathy with LVEF < 35% | 10 |
| Image indicating myxoma | 2 | 2 | 2 | Myxoma | 2 |
| Without structural heart disease | 336 | 0 | 61 | PFO with high embolic risk | 21 |
ASA, atrial septal aneurysm; LA, left atrium; LV, left ventricle; LVEF, left ventricular ejection fraction; LVH, left ventricular hypertrophy; PFO, patent foramen ovale; TEE, transesophageal echocardiography; TTE, transthoracic echocardiography.
Number of nonstructural heart diseases evaluated by TEE by clinical indication: ischemic stroke or transient ischemic attack of undetermined source after a complete etiological study in individuals younger than 60 years.
Table 3 shows the characteristics of the patients after the etiological study and the differences between patients with and without SHD.
Characteristics of the patients with stroke or TIA evaluated with focused cardiac ultrasound after an etiological study by the presence of structural heart disease
| Characteristic | Total (n = 706) | SHD(n = 368) | No SHD(n = 338) | P |
|---|---|---|---|---|
| Age, y | 66.8±13.2 | 69.1±13.2 | 64.3±12.8 | <.001 |
| Male sex | 430 (60.9) | 226 (61.4) | 204 (60.4) | .774 |
| Stroke etiology | ||||
| Cardioembolic | 182 (25.8) | 156 (42.4) | 26 (7.7) | <.001 |
| Atherothrombotic | 68 (9.6) | 23 (6.25) | 45 (13.3) | .002 |
| Undetermined | 323 (45.8) | 136 (36.9) | 187 (55.3) | <.001 |
| Lacunar | 114 (16.1) | 46 (12.5) | 68 (20.1) | .008 |
| Other determined cause | 19 (2.7) | 7 (1.9) | 12 (3.6) | .244 |
| Cardiac rhythm | ||||
| AF | 114 (16.1) | 91 (24.7) | 23 (6.8) | <.001 |
| Sinus | 585 (82.9) | 271 (73.6) | 314 (92.9) | <.001 |
| Pacemaker | 6 (0.8) | 5 (1.4) | 1 (0.3) | .219 |
| NIHSS at admission | 4.50±2.0 | 5.40±6.07 | 3.52±4.71 | <.001 |
| mRS at 3 mo ≥ 2 | 410 (58.1) | 225 (61.1) | 185 (54.7) | .093 |
AF, atrial fibrillation; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; SHD, structural heart disease.
Data are expressed as No. (%).
In 81.3% of patients with SHD, it was a de novo diagnosis (n = 299) without previous identification of the heart disease.
CVEs were analyzed from hospital discharge to the 1-year follow-up in patients with a de novo diagnosis of SHD vs patients without SHD. In total, 35 CVEs were recorded. Table 4 shows the frequencies of the different events and events subdivided by the presence of de novo SHD. CVEs were more frequent in patients with de novo SHD than in patients without SHD (8.36% vs 2.96%; P = .003).
Total cardiovascular events and types of cardiovascular events detected in patients with ischemic stroke or TIA studied with focused cardiac ultrasound by whether they had de novo structural heart disease or not
| Variable | Total (n = 637) | De novo SHD (n = 299) | Without SHD (n = 338) | P |
|---|---|---|---|---|
| Total cardiovascular events | 35 (5.49) | 25 (8.36) | 10 (2.96) | .003 |
| Ischemic stroke | 16 (2.51) | 10 (3.68) | 5 (1.48) | .127 |
| Acute myocardial infarction | 3 (0.47) | 3 (1.00) | 0 | .103 |
| Heart failure | 9 (1.41) | 6 (2.01) | 3 (0.89) | .318 |
| De novo AF | 7 (1.10) | 5 (1.67) | 2 (0.59) | .262 |
AF, atrial fibrillation; SHD, structural heart disease; TIA, transient ischaemic attack.
Data are expressed as No. (%).
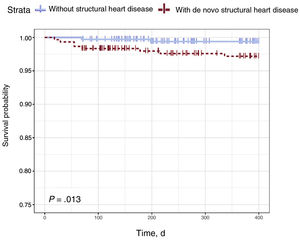
The Cox regression model revealed that the presence of de novo SHD was independently associated with the occurrence of CVEs (HR = 1.72; 95%CI, 1.01-2.91; P = .046). Age was also associated with CVEs (HR = 1.03; 95%CI, 1.01-1.05), whereas functional outcome at 3 months, measured using the modified Rankin Scale, exhibited an inverse association (HR = 0.46; 95%CI, 0.27-0.77) (table 5). Figure 2 shows 2 curves, namely, survival and disease-free survival until CVE occurrence, for patients with and without de novo SHD calculated using the Kaplan-Meier estimator. The evidence significantly indicated that survival probability due to CVEs is reduced in patients with de novo SHD (P = .013).
Results of the Cox regression model for factors associated with the development of cardiovascular events during follow-up
| Variable | Univariate Cox regression | |
|---|---|---|
| HR (95%CI) | P | |
| De novo SHD | 1.89 (1.13-3.19) | .016 |
| Age | 1.03 (1.01-1.05) | .006 |
| Sex | 1.23 (0.71-2.03) | .492 |
| AF | 1.33 (0.73-2.40) | .353 |
| NIHSS at admission | 0.99 (0.94-1.04) | .602 |
| mRS at 3 mo | 0.53 (0.32-0.90) | .017 |
| Cox multivariate regression | ||
| SHD | 1.72 (1.01-2.91) | .046 |
| Age | 1.03 (1.01-1.05) | .006 |
| mRS at 3 mo | 0.46 (0.27-0.77) | .003 |
95%CI, 95% confidence interval; AF, atrial fibrillation; HR, hazard ratio; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; SHD, structural heart disease.
The results of the present study show that focused cardiac ultrasound performed by qualified health care staff, in this case, neurologists, and integrated within a stroke care network with a cardiac imaging unit is a useful technique that permits the identification of a high number of patients with unknown SHD, who have a worse prognosis with a high incidence of CVEs during the first year of follow-up. This strategy enables the establishment of appropriate prevention strategies. In addition, combined with TEE and comprehensive TTE, this approach enables the detection of a high percentage of cardioembolic conditions, which permits a specific clinical and therapeutic management that, among other objectives, avoids new cerebral embolisms (figure 3).
As far as we know, this is the first description of the results of a focused cardiac ultrasound program conducted by neurologists in patients hospitalized for ischemic stroke or TIA and integrated in a network with a cardiac imaging unit. The digitalization and centralized storage of the medical images enabled the immediate and combined analysis of the images with a cardiac imaging specialist, which eliminated the need for a comprehensive echocardiogram in more than 90% of patients and can alleviate the health care demand in these units. In addition, the knowledge of specifically trained neurologists permits the correct interpretation of the pretest probability and etiology of the findings. This binomial integration of neurologist and cardiologist facilitates the decision-making process.
Other studies that analyzed the feasibility and diagnostic accuracy of this technique in the hands of noncardiologists vs comprehensive echocardiography also obtained excellent results.31–34
In our study, 38.3% of patients were found to have had an ischemic stroke or TIA due to an unknown cause or suspected cardioembolic etiology. According to the data obtained by a systematic review that analyzed the performance of TTE in patients who had a stroke or TIA, this approach was cost-effective only when the study was considered appropriate by the physicians,35 and the clinical practice guidelines, along the same lines, recommend TTE or TEE for patients with unidentified stroke or TIA and a suspected cardioembolic etiology.7
In our study, screening for SHD via systematic focused cardiac ultrasound in patients with ischemic stroke or TIA of undetermined etiology at admission or with suspected cardioembolic etiology detected abnormal findings in 52% of studies.
Based on the detection of SHD, which includes cardioembolic conditions, preventive measures have been implemented for new cerebral embolisms that could determine the absence of differences in the incidence of ischemic stroke in patients with and without SHD.
In addition, the various cardioembolic source findings also benefit from CVE prevention strategies, as in the case of moderate-to-severe left ventricular hypertrophy,36 left valvular heart diseases such as moderate-to-severe aortic or mitral regurgitation,37 or the detection of thoracic aortic dilatation.38
Our study contains one of the largest sample sizes in this field, and we analyzed the incidence of not only cardioembolic sources with direct implications for the secondary prevention of stroke, but those derived from the presence of SHD at a 1-year follow-up. SHD was associated with a higher long-term risk of CVEs, mainly congestive heart failure.
LimitationsThe main limitation of the study is that we did not analyze impact in terms of the need for and efficiency of the introduction of this technique into routine clinical practice.
In addition, focused cardiac ultrasound was not performed in all patients with ischemic stroke or TIA of unknown etiology at admission, which could represent a selection bias and underestimate the finding of heart diseases in patients with cerebrovascular disease.
In this study, we did not assess patients’ risk factors or other data from their medical records, such as the treatment received at the time of the ischemic stroke (intravenous fibrinolysis or mechanical thrombectomy) or the antithrombotic therapy prescribed at discharge. Another limitation is that this work constitutes a single-center study.
CONCLUSIONSFocused cardiac ultrasound performed and interpreted by accredited and trained professionals, in this case, neurologists, within a care network with cardiac imaging units is an accessible technique with high diagnostic yield. Its use permits clinical and therapeutic interventions for the prevention of new cerebral embolisms and other CVEs in stroke patients.
FUNDINGNo funding was received for the preparation of this manuscript.
AUTHORS’ CONTRIBUTIONSI. López-Dequidt, first author: design, patient enrollment, and article drafting. A. Martínez-Monzonis: design and article drafting. C. Peña-Gil: design and article drafting. A. González-Maestro: statistical analysis. V. González-Salvado: article drafting. M. Santamaría-Cadavid: patient enrollment and article drafting. S. Arias-Rivas: patient enrollment and article drafting. J.M. Prieto-González: coordination and article drafting. J.R. González-Juanatey, corresponding author: coordination, article design, and article drafting.
- –
In the etiological study of ischemic stroke, the systematic performance of TTE is recommended. A focused cardiac ultrasound training program has recently been introduced for neurologists.
- –
We analyzed for the first time the usefulness of this technique to identify heart diseases in stroke units coordinating with cardiac imaging units.
- –
The results show a high detection of structural heart disease, which was associated with worse long-term prognosis due to increased CVEs, and of cardioembolic sources, which required appropriate prevention strategies.
None.