Despite advances in treatment, patients with acute myocardial infarction (AMI) still exhibit unfavorable short- and long-term prognoses. In addition, there is scant evidence about the clinical outcomes of patients with AMI and coronavirus disease 2019 (COVID-19). The objective of this study was to describe the clinical presentation, complications, and risk factors for mortality in patients admitted for AMI during the COVID-19 pandemic.
MethodsThis prospective, multicenter, cohort study included all consecutive patients with AMI who underwent coronary angiography in a 30-day period corresponding chronologically with the COVID-19 outbreak (March 15 to April 15, 2020). Clinical presentations and outcomes were compared between COVID-19 and non-COVID-19 patients. The effect of COVID-19 on mortality was assessed by propensity score matching and with a multivariate logistic regression model.
ResultsIn total, 187 patients were admitted for AMI, 111 with ST-segment elevation AMI and 76 with non-ST-segment elevation AMI. Of these, 32 (17%) were diagnosed with COVID-19. GRACE score, Killip-Kimball classification, and several inflammatory markers were significantly higher in COVID-19-positive patients. Total and cardiovascular mortality were also significantly higher in COVID-19-positive patients (25% vs 3.8% [P <.001] and 15.2% vs 1.8% [P=.001], respectively). GRACE score> 140 (OR, 23.45; 95%CI, 2.52–62.51; P=.005) and COVID-19 (OR, 6.61; 95%CI, 1.82-24.43; P=.02) were independent predictors of in-hospital death.
ConclusionsDuring this pandemic, a high GRACE score and COVID-19 were independent risk factors associated with higher in-hospital mortality.
Keywords
Despite the widespread use of reperfusion techniques and the improvement in adjunctive medical therapies, patients with acute myocardial infarction (AMI) still face a substantial risk of further cardiovascular events and mortality.1,2 Thus, several risk factors and scores have been developed to predict both short- and long-term adverse outcomes.3–5
Since the devastating coronavirus disease 2019 (COVID-19) outbreak in early 2020, different types of cardiovascular involvement have been reported, such as acute coronary syndrome, myocarditis, takotsubo cardiomyopathy, cardiac arrest, and pulmonary thromboembolism.6–9 The potential mechanisms could include direct toxicity via internalization of the virus inside the myocyte through angiotensin-converting enzyme-2 membrane receptors, a supply-demand mismatch of oxygen in the context of sepsis, and a hypoxemia-mediated process due to acute respiratory distress syndrome (ARDS).10 All of these factors, besides producing myocardial injury, could also set the stage for coronary plaque destabilization, atherothrombosis, and AMI.
In addition, it is important to emphasize the current lack of knowledge about the clinical presentation, treatment, outcomes, and in-hospital mortality of patients with COVID-19 who develop AMI. Furthermore, it remains unclear whether COVID-19 is an independent predictor of mortality in the context of AMI. Accordingly, we compared mortality and hospital complications between a series of COVID-19 and non-COVID-19 patients with AMI undergoing coronary angiography during the outbreak period and investigated whether the presence of COVID-19 was an independent predictor of in-hospital mortality in patients with AMI during the pandemic.
METHODSStudy design and patient populationThis prospective, multicenter, observational cohort study was based on data obtained from the 7 tertiary Spanish hospitals with on-site percutaneous coronary intervention (PCI) capability operating a 24-hour, 7-day a week service (RECOVID-SCA Registry). This registry included all consecutive AMI patients with or without ST-segment elevation (STEMI or NSTEMI) who underwent coronary angiography from March 15 to April 15, 2020. This period chronologically corresponds with the COVID-19 surge in Spain. The study was performed in accordance with the 1964 Declaration of Helsinki and its subsequent amendments and was approved by the Ethics Committee from the hospital in charge of coordinating the registry. An independent academic clinical end point committee performed blinded adjudication of events.
Data collection and definitionsDemographic information, cardiovascular history, and risk factors were recorded: sex, body mass index, hypertension, diabetes mellitus, dyslipidemia, chronic kidney disease with creatinine clearance <30mL/min, smoking (current or former), chronic obstructive pulmonary disease, heart failure (both preserved and reduced ejection fraction), prior coronary artery disease, stroke or transient ischemic attack, any pattern of atrial fibrillation,11 cancer, and chronic anticoagulant treatment.
Patients were classified according to their initial diagnosis as STEMI or NSTEMI. STEMI was diagnosed in patients with clinical characteristics of myocardial ischemia and an electrocardiogram with ST-segment elevation in at least 2 contiguous leads.12 Meanwhile, NSTEMI diagnosis required the presence of angina associated with a cardiac biomarker increase above the 99th percentile of the upper reference limit.13 Patients who were finally diagnosed with myocarditis or takotsubo cardiomyopathy were excluded. Likewise, patients were classified according to whether they tested positive for COVID-19. This diagnosis was made in each center according to World Health Organization guidelines.14 In each patient, a real-time fluorescence polymerase chain reaction was performed to detect the positive nucleic acid of SARS-CoV-2 in throat swabs or in lower respiratory tract samples.
Clinical manifestations, electrocardiographic and echocardiographic findings, Killip-Kimball classification, and GRACE score were recorded, as well as the results of the following laboratory tests: cardiac troponin, creatine kinase, N-terminal pro-brain natriuretic peptide, brain natriuretic peptide, C-reactive protein, D-dimer, ferritin, complete blood count, and liver function. Each center measured cardiac troponin I or T using high-sensitivity assays with different upper reference limits. To standardize these results, we used the ratio of the observed troponin value divided by the cutoff points for the 99th percentile of the upper reference limit from every center, as performed previously.15
The following procedure-related findings were assessed: vascular access, number of coronary vessels with severe stenosis (> 70%), culprit vessel, angiographic characteristics (thrombus characterization according to the Thrombolysis in Myocardial Infarction [TIMI] study group grading system and the TIMI flow grade before and after the procedure),16,17 number of stents, and thrombus aspiration during PCI.
The following in-hospital adverse events were considered: bleeding according to the Bleeding Academic Research Consortium (BARC) scale,18 ARDS requiring tracheal intubation, stroke, systemic arterial thromboembolism, myocardial reinfarction, the use of mechanical circulatory support, sustained ventricular arrhythmias, death, and cardiovascular death. Re-infarction was considered when ST-segment elevation ≥ 1mm recurred or new pathognomonic Q waves appeared in at least 2 contiguous leads, particularly when associated with ischemic symptoms and troponin elevation (> 20% increase in the troponin value in the second sample).19 ARDS was defined as an acute condition characterized by bilateral pulmonary infiltrates and severe hypoxemia (ratio of the partial pressure of oxygen in the patient's arterial blood [PaO2] to the fraction of oxygen in inspired air [FiO2] in the absence of evidence of cardiogenic pulmonary edema.20 Cardiovascular deaths included deaths due to AMI, sudden cardiac death, death due to heart failure, death due to stroke, death due to cardiovascular hemorrhage, or death secondary to cardiovascular procedures.21 Embolism refers to systemic embolism and stroke and excludes venous thromboembolism.
Statistical analysisBaseline variables and clinical outcomes during admission were compared between patients with and without COVID-19 using a chi-square or Fisher exact test (if the expected value in each cell was less than 5) for categorical variables and a t test or Mann-Whitney U test (if the assumption of normality was significant [Shapiro-Wilk test P <.05]) for continuous variables, as appropriate. No imputation of missing data was required. In all tests, the P values of all outcomes were 2-sided; a value less than .05 was considered to indicate statistical significance. Confidence intervals were defined as 95% (95%CI). A multivariate logistic regression model was used to identify independent predictors of in-hospital death using “Forward: conditional” selection. Patients with type 2 AMI were excluded from this analysis due to its distinct pathophysiology vs type 1 AMI. Variables with P <.05 in univariate analysis were entered into a multivariate analysis. GRACE score> 140 and left ventricular ejection fraction <30% were analyzed as categorical variables. Results are reported as odds ratios (ORs) with associated 95%CIs. The Hosmer-Lemeshow test and the Nagelkerke R2 from the regression modeling were used as indicators of the goodness-of-fit of each risk model and to assess their calibration ability.
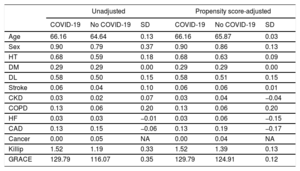
In addition, due to the observational nature of the study, there were some differences in the baseline characteristics between the patients in the 2 groups. Given these differences, a propensity score analysis was performed using generalized boosted models with COVID-19 as the dependent variable and the baseline characteristics outlined in table 1 as covariates. A propensity score-weighted analysis was performed to determine the effect of COVID-19 on mortality.
Covariates in propensity score analysis with the use of COVID-19 as the dependent variable
| Unadjusted | Propensity score-adjusted | |||||
|---|---|---|---|---|---|---|
| COVID-19 | No COVID-19 | SD | COVID-19 | No COVID-19 | SD | |
| Age | 66.16 | 64.64 | 0.13 | 66.16 | 65.87 | 0.03 |
| Sex | 0.90 | 0.79 | 0.37 | 0.90 | 0.86 | 0.13 |
| HT | 0.68 | 0.59 | 0.18 | 0.68 | 0.63 | 0.09 |
| DM | 0.29 | 0.29 | 0.00 | 0.29 | 0.29 | 0.00 |
| DL | 0.58 | 0.50 | 0.15 | 0.58 | 0.51 | 0.15 |
| Stroke | 0.06 | 0.04 | 0.10 | 0.06 | 0.06 | 0.01 |
| CKD | 0.03 | 0.02 | 0.07 | 0.03 | 0.04 | −0.04 |
| COPD | 0.13 | 0.06 | 0.20 | 0.13 | 0.06 | 0.20 |
| HF | 0.03 | 0.03 | −0.01 | 0.03 | 0.06 | −0.15 |
| CAD | 0.13 | 0.15 | −0.06 | 0.13 | 0.19 | −0.17 |
| Cancer | 0.00 | 0.05 | NA | 0.00 | 0.04 | NA |
| Killip | 1.52 | 1.19 | 0.33 | 1.52 | 1.39 | 0.13 |
| GRACE | 129.79 | 116.07 | 0.35 | 129.79 | 124.91 | 0.12 |
CAD, coronary artery disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; DL, dyslipidemia; DM, diabetes mellitus; GRACE, Global Registry of Acute Coronary Events; HF, heart failure; HT, hypertension; NA, not available; SD, standard deviation.
Analyses were performed with the software package IBM SPSS Statistics for Mac version 25 (IBM Corp, Armonk, New York, United States) and R statistics version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria).
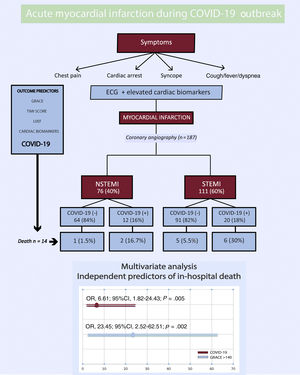
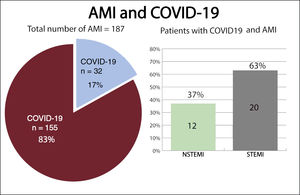
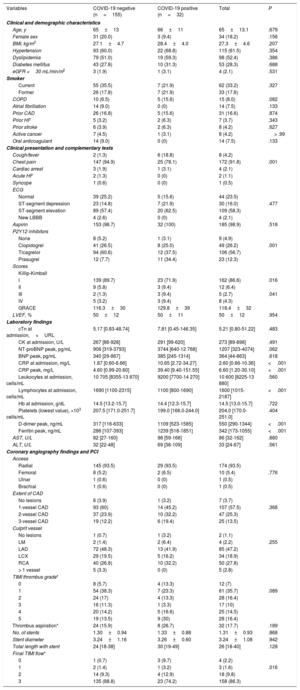
RESULTSPatient and baseline characteristicsBetween March 15, 2020, and April 15, 2020, 193 patients were diagnosed with AMI. Six patients were excluded: 3 who were diagnosed with myocarditis, 1 of which was fulminant myocarditis resulting in death, and 3 who were diagnosed with tako-tsubo cardiomyopathy. A total of 187 patients were included in the study, 76 with NSTEMI (40%) and 111 with STEMI (60%) (figure 1). The mean age at admission was 65±13 years old and 34 patients (18%) were female. Out of all patients, 32 (17%) tested positive for COVID-19: 12 (37%) had NSTEMI and 20 (63%) had STEMI (figure 2). Ten patients with no infectious symptoms did not undergo a real-time fluorescence polymerase chain reaction for SARS-CoV-2 and were considered negative for COVID-19 (8 with STEMI and 2 with NSTEMI). None of the patients had adverse events. There were no statistically significant differences in baseline characteristics between patients who were diagnosed with COVID-19 and those who were not (table 2).
Acute myocardial infarction during the COVID-19 outbreak. 95%CI, 95%confidence interval; COVID-19, coronavirus disease 2019; ECG, electrocardiogram; GRACE, Global Registry of Acute Coronary Events; LVEF, left ventricular ejection fraction; NSTEMI, non-ST-segment elevation myocardial infarction; OR, odds ratio; STEMI, ST-segment elevation myocardial infarction; TIMI, Thrombolysis in Myocardial Infarction.
Clinical characteristics, laboratory, procedures, and outcomes of AMI patients during the COVID-19 outbreak
| Variables | COVID-19 negative (n=155) | COVID-19 positive (n=32) | Total | P |
|---|---|---|---|---|
| Clinical and demographic characteristics | ||||
| Age, y | 65±13 | 66±11 | 65±13.1 | .679 |
| Female sex | 31 (20.0) | 3 (9.4) | 34 (18.2) | .156 |
| BMI, kg/m2 | 27.1±4.7 | 28.4±4.0 | 27.3±4.6 | .207 |
| Hypertension | 93 (60.0) | 22 (68.8) | 115 (61.5) | .354 |
| Dyslipidemia | 79 (51.0) | 19 (59.3) | 98 (52.4) | .386 |
| Diabetes mellitus | 43 (27.8) | 10 (31.3) | 53 (28.3) | .688 |
| eGFR <30 mL/min/m2 | 3 (1.9) | 1 (3.1) | 4 (2.1) | .531 |
| Smoker | ||||
| Current | 55 (35.5) | 7 (21.9) | 62 (33.2) | .327 |
| Former | 26 (17.8) | 7 (21.9) | 33 (17.6) | |
| COPD | 10 (6.5) | 5 (15.6) | 15 (8.0) | .082 |
| Atrial fibrillation | 14 (9.0) | 0 (0) | 14 (7.5) | .133 |
| Prior CAD | 26 (16.8) | 5 (15.6) | 31 (16.6) | .874 |
| Prior HF | 5 (3.2) | 2 (6.3) | 7 (3.7) | .343 |
| Prior stroke | 6 (3.9) | 2 (6.3) | 8 (4.2) | .627 |
| Active cancer | 7 (4.5) | 1 (3.1) | 8 (4.2) | > .99 |
| Oral anticoagulant | 14 (9.0) | 0 (0) | 14 (7.5) | .133 |
| Clinical presentation and complementary tests | ||||
| Cough/fever | 2 (1.3) | 6 (18.8) | 8 (4.2) | |
| Chest pain | 147 (94.9) | 25 (78.1) | 172 (91.8) | .001 |
| Cardiac arrest | 3 (1.9) | 1 (3.1) | 4 (2.1) | |
| Acute HF | 2 (1.3) | 0 (0) | 2 (1.1) | |
| Syncope | 1 (0.6) | 0 (0) | 1 (0.5) | |
| ECG | ||||
| Normal | 39 (25.2) | 5 (15.6) | 44 (23.5) | |
| ST-segment depression | 23 (14.8) | 7 (21.9) | 30 (16.0) | .477 |
| ST-segment elevation | 89 (57.4) | 20 (62.5) | 109 (58.3) | |
| New LBBB | 4 (2.6) | 0 (0) | 4 (2.1) | |
| Aspirin | 153 (98.7) | 32 (100) | 185 (98.9) | .518 |
| P2Y12 inhibitors | ||||
| None | 8 (5.2) | 1 (3.1) | 9 (4.9) | |
| Clopidogrel | 41 (26.5) | 8 (25.0) | 49 (26.2) | .001 |
| Ticagrelor | 94 (60.6) | 12 (37.5) | 106 (56.7) | |
| Prasugrel | 12 (7.7) | 11 (34.4) | 23 (12.3) | |
| Scores | ||||
| Killip-Kimball | ||||
| I | 139 (89.7) | 23 (71.9) | 162 (86.6) | .016 |
| II | 9 (5.8) | 3 (9.4) | 12 (6.4) | |
| III | 2 (1.3) | 3 (9.4) | 5 (2.7) | .041 |
| IV | 5 (3.2) | 3 (9.4) | 8 (4.3) | |
| GRACE | 116.3±30 | 129.8±39 | 118.4±32 | |
| LVEF, % | 50±12 | 50±11 | 50±12 | .954 |
| Laboratory findings | ||||
| cTn at admission,×URL | 5.17 [0.83-48.74] | 7.81 [0.45-146.35] | 5.21 [0.80-51.22] | .483 |
| CK at admission, U/L | 267 [86-928] | 291 [99-620] | 273 [89-898] | .491 |
| NT-proBNP peak, pg/mL | 906 [319-3793] | 3744 [640-12 768] | 1207 [323-4074] | .062 |
| BNP peak, pg/mL | 340 [29-867] | 385 [245-1314] | 364 [44-863] | .618 |
| CRP at admission, mg/L | 1.87 [0.60-6.66] | 10.65 [2.72-34.27] | 2.60 [0.66-10.36] | <.001 |
| CRP peak, mg/L | 4.60 [0.99-20.60] | 39.40 [9.40-151.55] | 6.60 [1.20-30.10] | <.001 |
| Leukocytes at admission, cells/mL | 10 705 [8355-13 870] | 9200 [7700-14 270] | 10 600 [8225-13 880] | .560 |
| Lymphocytes at admission, cells/mL | 1690 [1100-2315] | 1100 [800-1690] | 1600 [1015-2187] | <.001 |
| Hb at admission, g/dL | 14.5 [13.2-15.7] | 14.4 [12.3-15.7] | 14.5 [13.0-15.7] | .722 |
| Platelets (lowest value), ×103 cells/mL | 207.5 [171.0-251.7] | 199.0 [166.0-244.0] | 204.0 [170.0-251.0] | .404 |
| D-dimer peak, ng/mL | 317 [116-633] | 1109 [523-1585] | 550 [290-1344] | <.001 |
| Ferritin peak, ng/mL | 286 [107-393] | 1239 [518-1851] | 342 [173-1055] | <.001 |
| AST, U/L | 82 [27-160] | 98 [59-166] | 86 [32-162] | .880 |
| ALT, U/L | 32 [22-48] | 69 [38-109] | 33 [24-67] | .561 |
| Coronary angiography findings and PCI | ||||
| Access | ||||
| Radial | 145 (93.5) | 29 (93.5) | 174 (93.5) | |
| Femoral | 8 (5.2) | 2 (6.5) | 10 (5.4) | .776 |
| Ulnar | 1 (0.6) | 0 (0) | 1 (0.5) | |
| Brachial | 1 (0.6) | 0 (0) | 1 (0.5) | |
| Extent of CAD | ||||
| No lesions | 6 (3.9) | 1 (3.2) | 7 (3.7) | |
| 1-vessel CAD | 93 (60) | 14 (45.2) | 107 (57.5) | .368 |
| 2-vessel CAD | 37 (23.9) | 10 (32.2) | 47 (25.3) | |
| 3-vessel CAD | 19 (12.2) | 6 (19.4) | 25 (13.5) | |
| Culprit vessel | ||||
| No lesions | 1 (0.7) | 1 (3.2) | 2 (1.1) | |
| LM | 2 (1.4) | 2 (6.4) | 4 (2.2) | .255 |
| LAD | 72 (48.3) | 13 (41.9) | 85 (47.2) | |
| LCX | 29 (19.5) | 5 (16.2) | 34 (18.9) | |
| RCA | 40 (26.8) | 10 (32.2) | 50 (27.8) | |
| > 1 vessel | 5 (3.3) | 0 (0) | 5 (2.8) | |
| TIMI thrombus grade* | ||||
| 0 | 8 (5.7) | 4 (13.3) | 12 (7) | |
| 1 | 54 (38.3) | 7 (23.3) | 61 (35.7) | .089 |
| 2 | 24 (17) | 4 (13.3) | 28 (16.4) | |
| 3 | 16 (11.3) | 1 (3.3) | 17 (10) | |
| 4 | 20 (14.2) | 5 (16.6) | 25 (14.5) | |
| 5 | 19 (13.5) | 9 (30) | 28 (16.4) | |
| Thrombus aspiration* | 24 (15.9) | 8 (26.7) | 32 (17.7) | .189 |
| No. of stents | 1.30±0.94 | 1.33±0.88 | 1.31±0.93 | .868 |
| Stent diameter | 3.24±1.16 | 3.26±0.60 | 3.24±1.08 | .942 |
| Total length with stent | 24 [18-38] | 30 [19-49] | 26 [18-40] | .128 |
| Final TIMI flow* | ||||
| 0 | 1 (0.7) | 3 (9.7) | 4 (2.2) | |
| 1 | 2 (1.4) | 1 (3.2) | 3 (1.6) | .016 |
| 2 | 14 (9.3) | 4 (12.9) | 18 (9.8) | |
| 3 | 135 (88.8) | 23 (74.2) | 158 (86.3) | |
AMI, acute myocardial infarction, ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; BNP, brain natriuretic peptide; CAD, coronary artery disease; CK, total creatine kinase; COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; cTn, cardiac troponin; ECG, electrocardiogram; eGFR, estimated glomerular filtration rate; GRACE, Global Registry of Acute Coronary Events; Hb, hemoglobin; HF, heart failure; LAD, left anterior descending coronary artery; LBBB, left bundle branch block; LCX, left circumflex coronary artery; LM, left main coronary artery; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro-B-type natriuretic peptide; PCI, percutaneous coronary intervention; RCA, right coronary artery; STEMI, ST-segment elevation myocardial infarction; TIMI, Thrombolyis in Myocardial Infarction; ×URL, times the upper reference limit; SD, standard deviation.
Plus-minus values are means±standard deviation with a 95% confidence interval.
Data are expressed as No. (%), mean±standard deviation, or median [interquartile range].
Non-COVID-19 patients predominantly had chest pain, whereas COVID-19 patients mainly had respiratory symptoms such as fever and cough. Killip-Kimball III/IV and GRACE scores were significantly higher in patients with COVID-19. There were no significant differences in left ventricular ejection fraction between the 2 groups. COVID-19 patients had higher levels of C-reactive protein at admission and of peaks levels during hospitalization, fewer lymphocytes at admission, and higher peak levels of ferritin, D-dimer, and alanine aminotransferase. There were no significant differences in cardiac troponin at admission and in the peak measured during hospitalization between the 2 groups. Regarding antiplatelet treatment, patients with COVID-19 had a higher proportion of treatment with prasugrel. The hospital length of stay was significantly shorter in non-COVID-19 than COVID-19 patients (4.3±2.9 days vs 7.6±6.3 days, P <.01).
Coronary angiography and percutaneous coronary interventionsOf the 187 patients who underwent coronary angiography, 7 (3.7%) were finally diagnosed with type 2 AMI with nonobstructive coronary arteries; only 1 patient was COVID-19 positive (3.2%), whereas 6 were COVID-19 negative (3.8%). There were no differences in terms of mortality or major adverse cardiovascular events between patients with type 1 AMI or type 2 AMI.
Primary PCI was performed in 106 of the 111 patients with STEMI. The delay from symptom onset to guidewire crossing in STEMI patients was similar in COVID-19 and non-COVID-19 patients (240 [interquartile range, 157-315] minutes vs 241 [99-420] minutes, P=.80). There was no evidence of a higher amount of thrombus measured by TIMI thrombus score or a higher rate of thrombectomy in any of the groups.
The presence of multivessel coronary artery disease was similar in the 2 groups. There were no significant differences with respect to the treatment of nonculprit vessels during admission, with rates of 21% in COVID-19 patients and of 23% in non-COVID-19 patients (P=.20). We also found no differences in the anatomical characteristics of the culprit vessel or number of implanted stents. Procedure-related complications were also similar in the 2 groups.
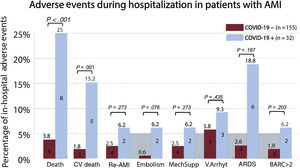
Outcomes and adverse eventsThere were 14 in-hospital deaths (7.5%, 95%CI, 4-11). Total mortality (8 [25%] vs 6 [3.8%], P <.001) and cardiovascular death (5 [15.2%] vs 3 [1.8%], P=.001) were significantly higher in the COVID-19 group than in the non-COVID-19 group (figure 3). No significant differences were found in the remaining adverse events. COVID-19 patients with AMI and bilateral pneumonia who needed mechanical ventilation because of ARDS had higher mortality than patients without ARDS (83% vs 12%, P=.002).
Association of COVID-19 with adverse events in patients admitted for acute myocardial infarction. AMI, acute myocardial infarction; ARDS, acute respiratory distress syndrome; BARC, Bleeding Academic Research Consortium scale; COVID-19, coronavirus disease 2019; CV death, cardiovascular death; Embolism, refers to stroke or systemic arterial embolism; MechSupp, mechanical circulatory support; Re-AMI, myocardial re-infarction; V.Arrhyt, ventricular arrhythmias.
The causes of death in COVID-19 patients were as follows: ARDS in 5 patients, cardiogenic shock after distal thrombus embolization from a left main coronary lesion in 2, and cardiac rupture as a mechanical complication of a STEMI in 1. In contrast, the causes of death in non-COVID-19 patients comprised 2 cardiogenic shocks, 1 possible stent thrombosis (Academic Research Consortium definition of stent thrombosis), 1 cerebellar stroke, 1 hypoxic encephalopathy after prolonged out-of-hospital cardiac arrest, and 1 fulminant hepatitis.
Risk factors for in-hospital mortalityUnivariate logistic regression analysis of the potential risk factors for total mortality is shown in . Risk factors for in-hospital mortality were age, Killip-Kimball> 2, GRACE score> 140, TIMI flow post-PCI <3, severe coronary calcification, left ventricular ejection fraction <30%, C-reactive protein, total creatine kinase, D-dimer, aspartate aminotransferase, ferritin, BARC> 2, ARDS, and COVID-19. When all of these variables were entered into a multivariate conditional logistic regression model, only COVID-19 (OR, 6.61; 95%CI, 1.82-24.43; P=.02) and GRACE score> 140 (OR, 23.45; 95%CI; 2.52-62.51; P=.005) remained independent predictors of in-hospital death (Hosmer and Lemeshow, P=.65; Nagelkerke, R2=0.54; figure 1). After propensity score matching, adequate comparability was achieved by a decrease in the standardized differences to less than 20% for all covariates (table 2). In the propensity score-adjusted analysis, mortality was significantly higher in the group of patients with COVID-19 (25.8% vs 18.0%, P=.045).
DISCUSSIONTo our knowledge, this is the first study to analyze the impact of COVID-19 on the prognosis of patients with AMI during the outbreak. The main finding is that COVID-19 is an independent factor related to in-hospital mortality in patients with AMI, in addition to the well-established GRACE score. Many multivariable prognostic models have been developed in populations of patients with STEMI5,22–24 and NSTEMI25,26 but none during the COVID-19 pandemic.
The large multinational observational Global Registry of Acute Coronary Events (GRACE) has also demonstrated excellent ability to assess the risk of in-hospital death.27 The most remarkable finding of this study is that COVID-19 was independently associated with higher in-hospital mortality in patients with AMI. If we analyze the possible reasons for this result, the first difference that we find is that patients with COVID-19 more frequently had atypical symptoms, particularly those suggestive of a respiratory infection, such as fever and cough. The delay in diagnosis may have resulted in later treatment, which would unavoidably worsen outcomes. Another aspect that might delay the diagnosis of AMI due to its atypical presentation is female sex. As in patients with COVID-19, AMI in women also has an atypical presentation (women experience symptoms other than typical chest pain, such as asthenia and dyspnea) and is associated with worse prognosis.28,29 Levels of the acute-phase reactant C-reactive protein were higher in the group with COVID-19. This protein reflects systemic and vascular inflammation and can predict future cardiovascular events. Elevated C-reactive protein is a predictor of adverse outcomes in patients with AMI and helps to identify patients who may be at risk of cardiovascular complications.26 Atherosclerosis is an inflammatory process, and plasma markers of inflammation are potential tools for the prediction of coronary events. Analysis by Chew et al.30 showed that C-reactive protein predicted the 30-day risk of death or myocardial infarction in patients undergoing PCI. C-reactive protein was found to be independently associated with the recurrence of cardiovascular events and with death in the mid- to long-term.31,32 It has been suggested that C-reactive protein may not only be a marker of generalized inflammation, but also directly and actively participate in both atherogenesis and atheromatous plaque disruption.33–35 Therefore, COVID-19 may predispose patients to thrombotic disease, both in the venous and arterial circulations, due to excessive inflammation, platelet activation, endothelial dysfunction, and stasis.36
There is a lack of information about the prognosis of AMI in patients with COVID-19. The recent series by Bangalore et al.37 showed that half of the patients underwent coronary angiography and that one-third of these patients had nonobstructive coronary artery disease. In our study of patients with AMI who underwent coronary angiography, the percentage of myocardial infarction with nonobstructive coronary arteries was similar. Myocardial injury in COVID-19 patients could be multifactorial, involving coronary plaque rupture and microthrombi, cytokine storm, coronary spasm, endothelial injury, and myocarditis or tako-tsubo cardiomyopathy. Considering that more than two-thirds of the COVID-19 patients with AMI in our series died of ARDS or fulminant myocarditis, the outcomes in these patients are determined by the severity of the COVID-19 pneumonia and the direct myocardial injury, with coronary thrombosis more a bystander than an actor in the disease process.
Our patients with COVID-19 were treated according to the guidelines, with all patients with STEMI undergoing primary PCI and all patients with NSTEMI receiving early invasive strategy.
LimitationsThere are several limitations to be considered in the interpretation of our study. First, this was an observational and nonrandomized study and, as such, the outcomes may have been influenced by both identified and unidentified confounders. Second, the number of patients studied with AMI and COVID-19 was small, which could limit the number of independent predictors identified and the consistency of the results. Finally, all variables were included via consultation of possibly incomplete medical records. Despite these limitations, the statistical significance was sufficient to allow us to draw preliminary conclusions.
CONCLUSIONSThis study shows that patients admitted for AMI with COVID-19 have higher risk scores, systemic inflammation, and higher in-hospital mortality. In addition, COVID-19 is an independent risk factor for in-hospital mortality, similar to a high GRACE score.
CONFLICTS OF INTERESTÁ. Sánchez-Recalde is Associate Editor of Rev Esp Cardiol. The journal's editorial procedure to ensure impartial handling of the manuscript has been followed.
- -
There is scant evidence on the clinical outcomes of patients with AMI and COVID-19. As far as we know, no series comparing COVID-19 and non-COVID-19 patients with AMI has been published.
- -
The risk factors for mortality in patients with AMI during the COVID-19 outbreak are also unknown.
- -
In patients diagnosed with AMI during the COVID-19 outbreak, COVID-19 was an independent predictor of in-hospital mortality, besides established factors such as a high GRACE score.
- -
Outcomes in these patients are determined by the severity of the COVID-19 disease and the direct myocardial injury. Meanwhile, coronary thrombosis is more of a bystander than an actor in the disease prognosis.
We are grateful to Dr Juan Manuel Monteagudo for his help with the methodological review of this manuscript.
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.rec.2020.07.009