There are limited data to develop a risk prediction model of in-hospital mortality for acute myocardial infarction (AMI) patients treated with venoarterial (VA)-extracorporeal membrane oxygenation (ECMO). We aimed to develop a risk prediction model for in-hospital mortality in patients with AMI who were treated with VA-ECMO.
MethodsA total of 145 patients with AMI who underwent VA-ECMO between May 2004 and April 2016 were included from the Samsung Medical Center ECMO registry. The primary outcome was in-hospital mortality. To develop a new predictive scoring system, named the AMI-ECMO score, backward stepwise elimination and β coefficient-based scoring were used based on logistic regression analyses. The leave-one-out cross-validation method was performed for internal validation.
ResultsIn-hospital mortality occurred in 69 patients (47.6%). On multivariable logistic regression analysis, the AMI-ECMO score comprised 6 pre-ECMO or angiographic parameters: age> 65 years, body mass index> 25 kg/m2, Glasgow coma score <6, lactic acid> 8 mmol/L, anterior wall infarction, and no or failed revascularization. The C-statistic value of AMI-ECMO score for predicting in-hospital mortality was 0.880 (95%CI, 0.820-0.940). The incidence of in-hospital mortality after VA-ECMO insertion was 6.2%, 28.1%, 51.6%, and 93.8% for AMI-ECMO score quartiles (0 to 16, 17 to 19, 20 to 26, and> 26), respectively (P <.001 for trend). The AMI-ECMO scores were also significantly associated with the estimated rate of all-cause mortality during follow-up (per 1 increase, HR, 1.11; 95%CI, 1.08-1.14; P <.001).
ConclusionsThe AMI-ECMO score can help predict early prognosis in AMI patients who undergo VA-ECMO.
Keywords
Despite advances in knowledge and treatment techniques for acute myocardial infarction (AMI), AMI complicated by cardiogenic shock (CS) is a still leading cause of death worldwide and a challenge for interventional cardiologists.1–3 Venoarterial-extracorporeal membrane oxygenation (VA-ECMO) can provide temporary mechanical full hemodynamic cardiopulmonary support until recovery of cardiac function in patients with CS refractory to conventional medical therapy.4–8 Based on several observational studies,9–14 VA-ECMO is a class IIb recommendation for AMI patients with refractory CS; however, large randomized controlled trials of the efficacy of VA-ECMO have not been performed and prognostic factors have not been fully elucidated.15,16 A recent study proposed a prediction risk model, named the ENCOURAGE score, for AMI patients who received VA-ECMO.17 However, the ENCOURAGE score is based on pre-ECMO factors only, not angiographic findings or peri-ECMO factors that are known to be prognostic factors in AMI patients. Therefore, our goal in this study was to identify predictors for in-hospital mortality in AMI patients treated with VA-ECMO and to develop a comprehensive risk prediction model including angiographic data.
METHODSStudy PopulationThe present study was a retrospective, single center, observational study of consecutive AMI patients who received VA-ECMO for refractory CS or cardiac arrest between May 2004 and April 2016 at Samsung Medical Center. Patients 18 years or older were included in this analysis. Venoarterial-ECMO was indicated in AMI patients with cardiac arrest or acute refractory cardiovascular failure, defined as evidence of organ hypoperfusion (extensive skin mottling, progressive lactic acidosis, oliguria or altered mental status) despite adequate intravascular volume management and maximal medical therapy including vasopressors or inotropes. All patients were expected to undergo early revascularization and to receive optimal medical therapy in accordance with current guidelines.15,16 A total of 145 patients with AMI who received VA-ECMO were finally eligible for this study. The final decision to implant VA-ECMO was determined by an experienced team, and the VA-ECMO was inserted at the bedside or in a catheterization laboratory by cardiovascular surgeons or interventional cardiologists. The requirement for informed consent was waived by the Institutional Review Board of Samsung Medical Center because of the retrospective nature of the study.
Implantation of the Venoarterial-extracorporeal Membrane Oxygenation Device and ManagementDetails of the management of VA-ECMO for AMI patients have been previously documented.18 In brief, a VA-ECMO device was inserted by percutaneous cannulation using the Seldinger technique or surgical cannulation using the cut-down method. Arterial cannula sized 14-Fr to 17-Fr and venous cannula sized 20-Fr to 24-Fr were used. Femoral vessels were usually used as vascular access sites. Capiox Emergency Bypass System (Capiox EBS; Terumo, Inc, Tokyo, Japan) and Permanent Life Support (PLS; MAQUET, Rastatt, Germany) were available in our hospital at the time of the study. In the event of distal limb ischemia after arterial cannulation, a catheter was inserted distal to the cannulation site for limb perfusion. Pump speed was adjusted to obtain a cardiac index greater than 2.2 L/min/body surface area (m2), mean arterial pressure> 65mmHg, and central mixed venous saturation> 70%. Intravenous heparin was infused to maintain an activated clotting time ranging from 150 to 180seconds unless life-threatening bleeding was observed. Successful weaning was defined as disconnection of the patient from ECMO without reinsertion or death within 24hours.
Data Collection and OutcomesThe following information was collected retrospectively through medical record review: age, sex, physical examination results, underlying comorbidities, angiographic data, laboratory data, echocardiography data, in-hospital management, and pre- and post-ECMO data. When the same laboratory data were measured several times before ECMO insertion, the laboratory value measured at the nearest time to ECMO insertion was recorded. When the neurologic evaluation was available before ECMO insertion, the Glasgow coma score was obtained at the nearest time to ECMO insertion time. However, if the patient was unable to perform a neurologic evaluation due to unexplained or out-of-hospital cardiac arrest, the Glasgow coma score was calculated after ECMO insertion. Scores from SAVE, and ENCOURAGE, which are previously validated scoring systems for evaluating survival to discharge in patients undergoing VA-ECMO, were calculated for comparison with the newly generated risk prediction model.17,19 Additional clinical information including follow-up data were obtained from medical records and telephone interviews by trained study coordinators if necessary. The median follow-up duration was 33.0 days [interquartile range from 4.0 to 373.0 days]. The primary outcome was in-hospital mortality, while the secondary outcome was all-cause mortality during the follow-up period.
Statistical AnalysisCategorical variables are presented as numbers and relative frequencies and their group differences were compared using the chi-square test or the Fisher exact test, as appropriate. Continuous variables are presented as mean±standard deviations or medians (25th-75th percentiles) and their group differences were compared using the Student t test. Multivariable logistic regression analysis was used to identify predictors of in-hospital mortality in patients with AMI who underwent VA-ECMO implantation. For practical utility purposes, continuous variables were transformed into categorical variables, which were assessed by normal range or best cutoff values in the maximally selected minimum P value method. Covariates that were considered clinically relevant or that showed a univariate relationship with outcome (P ≤ .2) were entered into the multivariable logistic regression model. Clinically intercorrelated variables were excluded from the model. Then, stepwise elimination analysis was performed to identify a useful subset of predictors. The AMI-ECMO risk score was then constructed to predict in-hospital mortality using a regression coefficient-based scoring method. To generate a simple integer-based point score for each predictive variable, each β coefficient was divided by the absolute value of the smallest coefficient, multiplied by 5, and rounded to the nearest integer. The discriminating power of the constructed score in predicting the in-hospital mortality was assessed by considering the area under the curve from the receiver operating characteristic (ROC) analysis. The adequacy of the model was checked using the Hosmer-Lemeshow goodness-of-fit test, and leave-one-out cross-validation was used for internal validation to obtain the misclassification error rate. To evaluate the association between AMI-ECMO score and estimated risk of all-cause mortality, the probability of death was estimated using the Cox proportional hazards model. The estimated risk of all-cause mortality was depicted using a locally weighted scatterplot smoothing regression line. The property of AMI-ECMO score was then compared with those of the ENCOURAGE and SAVE scores. Event-free survival according to the AMI-ECMO score quartiles was evaluated by Kaplan-Meier analysis, and the significance level was assessed with a log-rank test. Correlations among AMI-ECMO, ENCOURAGE, and SAVE scores were assessed with the Spearman correlation coefficient. Statistical analyses were performed using R Statistical Software (version 3.2.5; R Foundation for Statistical Computing, Vienna, Austria) with P <.05 considered statistically significant.
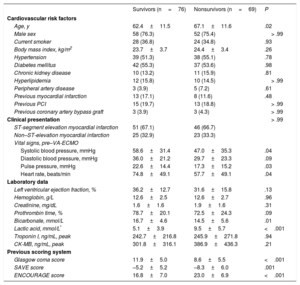
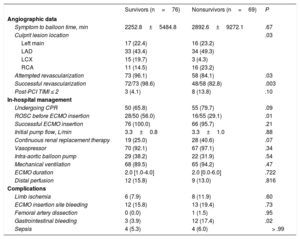
RESULTSStudy PopulationsAmong 145 AMI patients treated with VA-ECMO, 97 (66.9%) patients presented with ST-segment elevation myocardial infarction. Successful ECMO weaning was achieved in 91 patients (62.8%), and 76 patients (52.4%) survived to discharge. The median duration of mechanical support was 2.0 days [interquartile range: 1.0-4.0]. Table 1 shows the baseline clinical and laboratory characteristics of the participants, and Table 2 shows angiographic findings and provides in-hospital management information. Compared with survivors, nonsurvivors were older and had a lower systolic blood pressure, pulse pressure, heart rate, Glasgow coma score, proportion of successful revascularization, and lower return of spontaneous circulation before ECMO insertion. The incidence of gastrointestinal bleeding and lactic acid values were significantly higher in nonsurvivors than in survivors. Among the study population, only 1 patient received heart transplantation during VA-ECMO maintenance. However, heart transplantation was performed in 6 patients during follow-up because of aggravation of ischemic cardiomyopathy after AMI.
Baseline Characteristics
| Survivors (n=76) | Nonsurvivors (n=69) | P | |
|---|---|---|---|
| Cardiovascular risk factors | |||
| Age, y | 62.4±11.5 | 67.1±11.6 | .02 |
| Male sex | 58 (76.3) | 52 (75.4) | > .99 |
| Current smoker | 28 (36.8) | 24 (34.8) | .93 |
| Body mass index, kg/m2 | 23.7±3.7 | 24.4±3.4 | .26 |
| Hypertension | 39 (51.3) | 38 (55.1) | .78 |
| Diabetes mellitus | 42 (55.3) | 37 (53.6) | .98 |
| Chronic kidney disease | 10 (13.2) | 11 (15.9) | .81 |
| Hyperlipidemia | 12 (15.8) | 10 (14.5) | > .99 |
| Peripheral artery disease | 3 (3.9) | 5 (7.2) | .61 |
| Previous myocardial infarction | 13 (17.1) | 8 (11.6) | .48 |
| Previous PCI | 15 (19.7) | 13 (18.8) | > .99 |
| Previous coronary artery bypass graft | 3 (3.9) | 3 (4.3) | > .99 |
| Clinical presentation | > .99 | ||
| ST-segment elevation myocardial infarction | 51 (67.1) | 46 (66.7) | |
| Non–ST-elevation myocardial infarction | 25 (32.9) | 23 (33.3) | |
| Vital signs, pre–VA-ECMO | |||
| Systolic blood pressure, mmHg | 58.6±31.4 | 47.0±35.3 | .04 |
| Diastolic blood pressure, mmHg | 36.0±21.2 | 29.7±23.3 | .09 |
| Pulse pressure, mmHg | 22.6±14.4 | 17.3±15.2 | .03 |
| Heart rate, beats/min | 74.8±49.1 | 57.7±49.1 | .04 |
| Laboratory data | |||
| Left ventricular ejection fraction, % | 36.2±12.7 | 31.6±15.8 | .13 |
| Hemoglobin, g/L | 12.6±2.5 | 12.6±2.7 | .96 |
| Creatinine, mg/dL | 1.6±1.6 | 1.9±1.6 | .31 |
| Prothrombin time, % | 78.7±20.1 | 72.5±24.3 | .09 |
| Bicarbonate, mmol/L | 16.7±4.6 | 14.5±5.6 | .01 |
| Lactic acid, mmol/L* | 5.1±3.9 | 9.5±5.7 | <.001 |
| Troponin I, ng/mL, peak | 242.7±216.8 | 245.9±271.8 | .94 |
| CK-MB, ng/mL, peak | 301.8±316.1 | 386.9±436.3 | .21 |
| Previous scoring system | |||
| Glasgow coma score | 11.9±5.0 | 8.6±5.5 | <.001 |
| SAVE score | –5.2±5.2 | –8.3±6.0 | .001 |
| ENCOURAGE score | 16.8±7.0 | 23.0±6.9 | <.001 |
CK-MB, creatine kinase-ixoenzyme MB; PCI, percutaneous coronary intervention; VA-ECMO, venoarterial extracorporeal membrane oxygenation.
Values are mean±standard deviation or No. (%).
Baseline Angiographic Findings and In-hospital Management
| Survivors (n=76) | Nonsurvivors (n=69) | P | |
|---|---|---|---|
| Angiographic data | |||
| Symptom to balloon time, min | 2252.8±5484.8 | 2892.6±9272.1 | .67 |
| Culprit lesion location | .03 | ||
| Left main | 17 (22.4) | 16 (23.2) | |
| LAD | 33 (43.4) | 34 (49.3) | |
| LCX | 15 (19.7) | 3 (4.3) | |
| RCA | 11 (14.5) | 16 (23.2) | |
| Attempted revascularization | 73 (96.1) | 58 (84.1) | .03 |
| Successful revascularization | 72/73 (98.6) | 48/58 (82.8) | .003 |
| Post-PCI TIMI ≤ 2 | 3 (4.1) | 8 (13.8) | .10 |
| In-hospital management | |||
| Undergoing CPR | 50 (65.8) | 55 (79.7) | .09 |
| ROSC before ECMO insertion | 28/50 (56.0) | 16/55 (29.1) | .01 |
| Successful ECMO insertion | 76 (100.0) | 66 (95.7) | .21 |
| Initial pump flow, L/min | 3.3±0.8 | 3.3±1.0 | .88 |
| Continuous renal replacement therapy | 19 (25.0) | 28 (40.6) | .07 |
| Vasopressor | 70 (92.1) | 67 (97.1) | .34 |
| Intra-aortic balloon pump | 29 (38.2) | 22 (31.9) | .54 |
| Mechanical ventilation | 68 (89.5) | 65 (94.2) | .47 |
| ECMO duration | 2.0 [1.0-4.0] | 2.0 [0.0-6.0] | .722 |
| Distal perfusion | 12 (15.8) | 9 (13.0) | .816 |
| Complications | |||
| Limb ischemia | 6 (7.9) | 8 (11.9) | .60 |
| ECMO insertion site bleeding | 12 (15.8) | 13 (19.4) | .73 |
| Femoral artery dissection | 0 (0.0) | 1 (1.5) | .95 |
| Gastrointestinal bleeding | 3 (3.9) | 12 (17.4) | .02 |
| Sepsis | 4 (5.3) | 4 (6.0) | > .99 |
CPR, cardiopulmonary resuscitation; ECMO, extracorporeal membrane oxygenation; LAD, left anterior descending; LCX, left circumflex; PCI, percutaneous coronary intervention; RCA, right coronary artery; ROSC, return to spontaneous circulation; TIMI, Thrombolysis In Infarction. Values are expressed as mean ± standard deviation, No. (%), or No. [interquartile range].
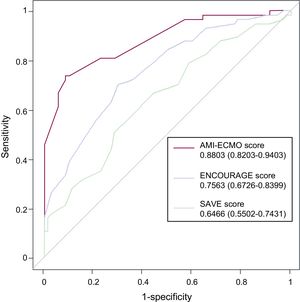
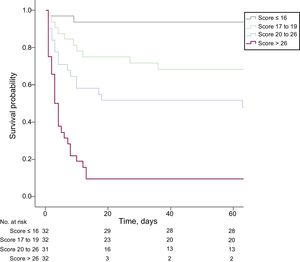
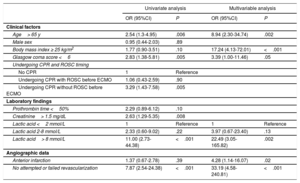
After continuous variables were transformed into categorical variables, independent predictors of in-hospital mortality were age> 65 years, body mass index> 25 kg/m2, Glasgow coma score <6, lactic acid> 8 mmol/L, anterior wall infarction, and no attempted or failed revascularization, based on multivariable analysis with adjustment for prognostic covariates (Table 3). The AMI-ECMO score was then generated to predict in-hospital mortality in AMI patients who received VA-ECMO. A full description of the model is provided in Table 4. The incidence of in-hospital mortality after VA-ECMO device implantation was 6.2%, 28.1%, 51.6%, and 93.8% for AMI-ECMO scores 0 to 16, 17 to 19, 20 to 26, and> 26 (divided by quartiles), respectively (P <.001 for trend). In addition, the AMI-ECMO score had good predictive ability as assessed by the area under the curve in ROC curve analysis (C-statistic 0.880; 95% confidence interval [95%CI], 0.820-0.940) with satisfactory calibration (Hosmer-Lemeshow chi-square=7.484, df=8; P=.485) (Figure 1). Compared with previously validated scoring systems, such as ENCOURAGE or SAVE, the AMI-ECMO score had the highest predictive ability in the present study population (C-statistic for AMI-ECMO score vs ENCOURAGE score, 0.880 vs 0.756; P=.003, ROC and AMI-ECMO score vs SAVE score 0.880 vs 0.647; P <.001, respectively). Correlations among scoring systems are shown in and demographic differences between AMI-ECMO and ENCOURAGE are shown in the . Leave-one-out cross-validation (analysis of the AMI-ECMO score for internal validation showed that it had the lowest misclassification error rate of in-hospital mortality of 14% compared with 26% and 38% for ENCOURAGE and SAVE, respectively.
Univariate and Multivariate Analyses of Factors Associated With In-hospital Mortality
| Univariate analysis | Multivariable analysis | |||
|---|---|---|---|---|
| OR (95%CI) | P | OR (95%CI) | P | |
| Clinical factors | ||||
| Age> 65 y | 2.54 (1.3-4.95) | .006 | 8.94 (2.30-34.74) | .002 |
| Male sex | 0.95 (0.44-2.03) | .89 | ||
| Body mass index ≥ 25 kg/m2 | 1.77 (0.90-3.51) | .10 | 17.24 (4.13-72.01) | <.001 |
| Glasgow coma score <6 | 2.83 (1.38-5.81) | .005 | 3.39 (1.00-11.46) | .05 |
| Undergoing CPR and ROSC timing | ||||
| No CPR | 1 | Reference | ||
| Undergoing CPR with ROSC before ECMO | 1.06 (0.43-2.59) | .90 | ||
| Undergoing CPR without ROSC before ECMO | 3.29 (1.43-7.58) | .005 | ||
| Laboratory findings | ||||
| Prothrombin time <50% | 2.29 (0.89-6.12) | .10 | ||
| Creatinine> 1.5 mg/dL | 2.63 (1.29-5.35) | .008 | ||
| Lactic acid <2 mmol/L | 1 | Reference | 1 | Reference |
| Lactic acid 2-8 mmol/L | 2.33 (0.60-9.02) | .22 | 3.97 (0.67-23.40) | .13 |
| Lactic acid> 8 mmol/L | 11.00 (2.73-44.38) | <.001 | 22.49 (3.05-165.82) | .002 |
| Angiographic data | ||||
| Anterior infarction | 1.37 (0.67-2.78) | .39 | 4.28 (1.14-16.07) | .02 |
| No attempted or failed revascularization | 7.87 (2.54-24.38) | <.001 | 33.19 (4.58-240.81) | <.001 |
95%CI, 95% confidence interval; CPR, cardiopulmonary resuscitation; ECMO, extracorporeal membrane oxygenation; OR, odds ratio; ROSC, return to spontaneous circulation.
Predicting Scoring System of In-hospital Mortality
| β-coefficient | OR | 95%CI | P | Score | ||
|---|---|---|---|---|---|---|
| Clinical factors | Age> 65 y | 2.39 | 10.97 | 2.95-40.72 | <.001 | 10 |
| Body mass index> 25 kg/m2 | 2.64 | 13.95 | 3.64-53.40 | <.001 | 11 | |
| Glasgow coma score <6 | 1.22 | 3.42 | 1.05-11.15 | .04 | 5 | |
| Laboratory findings | Lactic acid> 8 mmol/L | 2.23 | 9.26 | 2.77-31.01 | <.001 | 9 |
| Angiographic data | Anterior wall infarction | 1.79 | 5.98 | 1.66-21.47 | .006 | 7 |
| No attempted or failed revascularization | 3.38 | 29.36 | 4.67-184.42 | <.001 | 14 |
95%CI, 95% confidence interval; OR, odds ratio.
Lactic acid entered as a binary variable in the multivariable model.
Receiver operating characteristic curves of AMI-ECMO, ENCOURAGE, and SAVE scores for predicting in-hospital mortality. Receiver operating characteristic curves for predicting in-hospital mortality based on AMI-ECMO, ENCOURAGE, and SAVE scores are presented. Among the models, the AMI-ECMO score showed the highest C-statistic value. AMI, acute myocardial infarction; ECMO, extracorporeal membrane oxygenation.
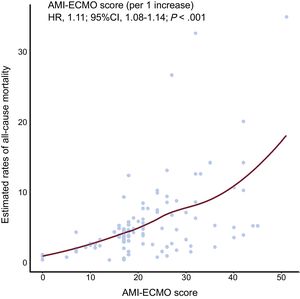
Among the patients who survived to discharge (76 patients), 8 patients died, 1 patient was lost to follow-up, and 68 patients were still alive during the follow-up period. AMI -ECMO scores as continuous values showed a significant association with the estimated rate of all-cause mortality (per 1 increase, hazard ratio, 1.11; 95%CI, 1.08-1.14, P <.001) (Figure 2). Comparison of 60-day all-cause mortality rates among the 4 groups, classified according to AMI-ECMO score quartiles, are shown in Figure 3. There were significant differences in all-cause mortality among the 4 groups (log-rank P <.001).
Estimated all-cause mortality rates according to AMI-ECMO score. The locally weighted scatterplot smoothing curve depicts correlations between the probability of all-cause mortality, which was estimated using a Cox proportional hazards regression model, and the AMI-ECMO score. The AMI-ECMO score was significantly correlated with estimated rate of all-cause mortality. 95%CI, 95% confidence interval; AMI, acute myocardial infarction; ECMO, extracorporeal membrane oxygenation; HR, hazard ratio.
Comparison of all-cause mortality according to AMI-ECMO score quartiles. Kaplan-Meier curve of 60-day survival probability according to AMI-ECMO score quartiles (≤ 16, 17 to 19, 20 to 26,> 26) is plotted. AMI, acute myocardial infarction; ECMO, extracorporeal membrane oxygenation.
In the present study, we identified independent predictors of in-hospital mortality and created an in-hospital mortality prediction score comprising 6 comprehensive patient characteristics: age, body mass index, Glasgow coma score, lactate, anterior wall infarction, and revascularization, in AMI patients who underwent VA-ECMO for refractory CS. The newly developed score, the AMI-ECMO score, not only had a good discrimination property of in-hospital mortality, but also showed a higher C-index for prediction of in-hospital mortality than previously validated VA-ECMO risk scores. Furthermore, AMI-ECMO score quartiles were significantly positively correlated with all-cause mortality during follow-up.
Wide use of early revascularization and improvement of evidence-based medical therapy have led to a marked decline in mortality due to AMI complicated by CS over the last 2 decades.20–23 However, refractory CS still remains the leading cause of death; overall survival was reported to be about 50% in patients hospitalized with AMI complicated by CS who underwent VA-ECMO.24 Consistent with previous studies, 47.6% of patients died in-hospital in our study. Additionally, inappropriate VA-ECMO device insertion may be associated with high complication rates, raised hospital costs, and unnecessary extension of therapy without sufficient mortality benefit.25 In light of this, initiation and maintenance of VA-ECMO should be carefully decided by risk-benefit analysis performed by experts. To date, 2 representative studies, the SAVE and ENCOURAGE studies, have been conducted to develop mortality prediction models in patients undergoing VA-ECMO.17,19 Nevertheless, there have been several limitations to the application of these models to patients with AMI in real-world practice. The SAVE score is very complex to use quickly and is difficult to apply to an AMI-specific population because the score was created based on patients with all-cause CS. Although the ENCOURAGE score was designed for AMI-specific populations being treated with VA-ECMO and comprises 6 simple variables, angiographic data such as successful revascularization or location of the culprit lesion, which is one of the most important treatment strategies for AMI, are not included in the ENCOURAGE score. Although the disease severity of the study population may have been slightly different than that in the current study, the recently documented substudy of the IABP-SHOCK II trial reported that angiographic findings, such as Thrombolysis In Myocardial Infarction flow grade <3 after percutaneous coronary intervention, were independent predictors of in-hospital mortality in patients with AMI-related CS.26 Therefore, we developed and analyzed a simple prediction model that includes angiographic and pre-ECMO variables to predict in-hospital mortality in AMI patients undergoing VA-ECMO.
A number of previous observational studies have identified independent predictors of in-hospital mortality in AMI patients with CS who underwent VA-ECMO.27–31 In the present study, independent predictors of in-hospital mortality were old age, obesity, low Glasgow coma score, elevated serum lactate level, anterior wall infarction, and unsuccessful revascularization. Pre-ECMO clinical and laboratory variables of old age, obesity, low Glasgow coma score, and elevated lactate were independent predictors in both our study and the ENCOURAGE study, which had the same design and population as our study. However, in contrast to the ENCOURAGE study, angiographic variables, including anterior location of the culprit lesion and unsuccessful revascularization, significantly predicted in-hospital mortality in the present study. These findings lend support to the finding in previous studies that anterior wall infarction is associated with a poor prognosis, and emphasize the importance of early revascularization in AMI patients with CS.29–32 Furthermore, the predictive ability for in-hospital mortality was numerically higher in the AMI-ECMO score (C-statistic 0.880) than in the ENCOURAGE score (C-statistic 0.840). However, neither of these studies have been validated in other cohorts. Therefore, future studies are needed for external validation and to compare the performance of these scores.
LimitationsOur study has several limitations. First, due to the retrospective nature of our database, some variables including lactic acid level, echocardiographic parameters, hemodynamic parameters, and neurologic data were not recorded for all patients. In particular, serum lactate data were available for only 87.6% (127/145) of the patients. Therefore, cases with missing value of serum lactate were excluded from the multivariable logistic regression model. Second, the AMI-ECMO score needs to be prospectively confirmed in other patient populations with AMI complicated by CS treated with VA-ECMO, because we performed only an internal validation of the AMI-ECMO score. Finally, this study was based on a single-center experience, which may limit the generalizability of our results and have created selection bias in the patients’ subset. Also, the relatively small sample size could have limited the precision of the estimates.
CONCLUSIONSUsing age, body mass index, Glasgow coma score, lactate, anterior wall infarction, and unsuccessful revascularization, we created a good risk prediction model for survival to discharge in AMI patients treated with VA-ECMO. The AMI-ECMO score will aid in decision-making in AMI complicated by CS assisted by VA-ECMO.
CONFLICTS OF INTERESTNone declared.
- –
Initiation and maintenance of VA-ECMO for AMI patients with refractory CS should be carefully decided by risk-benefit analysis assessed by expert.
- –
There are limited data to develop a risk prediction model of in-hospital mortality for AMI patients treated with VA-ECMO.
- –
The AMI-ECMO score, which incorporates age, body mass index, Glasgow coma score, lactate, anterior wall infarction, and revascularization, can help in decision-making for CS complicating AMI assisted by VA-ECMO and has good discrimination for in-hospital mortality.