Angiogenesis helps to reestablish microcirculation after myocardial infarction (MI). In this study, we aimed to further understand the role of the antiangiogenic isoform vascular endothelial growth factor (VEGF)-A165b after MI and to explore its potential as a coadjuvant therapy to coronary reperfusion.
MethodsTwo mice MI models were formed: a) permanent coronary ligation (nonreperfused MI); b) transient 45-minute coronary occlusion followed by reperfusion (reperfused MI); in both models, animals underwent echocardiography before euthanasia at day 21 after MI induction. We determined serum and myocardial VEGF-A165b levels. In both experimental MI models, we assessed the functional and structural role of VEGF-A165b blockade. In a cohort of 104 ST-segment elevation MI patients, circulating VEGF-A165b levels were correlated with cardiovascular magnetic resonance-derived left ventricular ejection fraction at 6 months and with the occurrence of adverse events (death, heart failure, and/or reinfarction).
ResultsIn both models, circulating and myocardial VEGF-A165b levels were increased 21 days after MI induction. Serum VEGF-A165b levels inversely correlated with systolic function evaluated by echocardiography. VEGF-A165b blockade increased capillary density, reduced infarct size, and enhanced left ventricular function in reperfused, but not in nonreperfused, MI experiments. In patients, higher VEGF-A165b levels correlated with depressed ejection fraction and worse outcomes.
ConclusionsIn experimental and clinical studies, higher serum VEGF-A165b levels are associated with worse systolic function. Their blockade enhances neoangiogenesis, reduces infarct size, and increases ejection fraction in reperfused, but not in nonreperfused, MI experiments. Therefore, VEGF-A165b neutralization represents a potential coadjuvant therapy to coronary reperfusion.
Keywords
Despite complete reperfusion at the epicardial level after myocardial infarction (MI), deterioration of myocardial perfusion can persist in more than 50% of patients. This phenomenon is known as microvascular obstruction and has deleterious structural and prognostic effects.1,2 To further minimize microvascular damage, it is mandatory to evaluate new coadjuvant therapies to early reperfusion.
Angiogenesis is involved in the repair process post-MI by reestablishing microvascular circulation, providing oxygen and nutrients to the cardiac tissue.3 In the MI scenario, the concentration of proangiogenic vascular endothelial growth factor (VEGF)-A was increased in plasma and the infarcted myocardium soon after MI induction.4 VEGF-A can be differentially spliced to generate the antiangiogenic VEGF-A165b isoform.5 In a cohort of 50 ST-segment elevation MI (STEMI) patients, serum VEGF-A165b levels were reported to increase post-MI and were associated with impaired cardiac structure determined by cardiovascular magnetic resonance (CMR) at first week after MI6. However, to date, the effects of VEGF-A165b activity in experimental models of MI and the potential effects of its blockade are still unknown.
To gain further insights into the role of VEGF-A165b post-MI and explore its potential as a coadjuvant therapy to early coronary reperfusion, the specific objectives of the present study were as follows. In 2 mice experimental MI models (nonreperfused and reperfused MI): a) to determine the role of VEGF-A165b in serum and in the infarcted myocardium; and b) to investigate in vivo the potential effects of VEGF-A165b neutralization on capillary density, infarct size, and systolic function. In a prospective cohort of 104 STEMI patients: c) to evaluate circulating VEGF-A165b levels, their association with the resultant cardiac structure evaluated at 6 months by CMR, and the occurrence of major adverse cardiac events (MACE) during follow-up.
METHODSAnimal studiesThe animal protocols were performed following the guidelines of Directive 2010/63/EU of the European Parliament and were approved by the ethics review board committee (Protocol number: 2016/VCS/PEA/00075).
C57BL/6J mice were supplied by Charles River Laboratories (Chatillon-sur-Chalaronne, France). Mice (age, 16±2 weeks) were bred and maintained under specific pathogen-free conditions at a constant temperature of 22±2°C and humidity of 60%-65% with a 12-hour dark/light cycle, and with free access to normal chow and autoclaved water.
Mouse MI modelMI was induced in mice (n=60) by ligation of the left anterior descending coronary artery, but 8 of them died prior to sacrifice. The mice were divided into 2 MI models: a) nonreperfused MI (permanent coronary ligation, without reperfusion); and b) reperfused MI (transient 45-minute occlusion of the coronary artery followed by reperfusion). In the nonreperfused MI model, the knot was tightened with the consequent occlusion of the coronary artery. In the reperfused MI model, a 23G tube was placed between the coronary artery and the 6-0 silk suture for later removal to allow complete reperfusion following the ischemic period. The tube was positioned through the sixth intercostal space to the exterior of the animal. In both models, once ischemia onset was confirmed, both visually and in an electrocardiogram, the mice thorax was closed. In the reperfused MI model, the tube was removed after 45minutes of occlusion to allow coronary reperfusion, which was confirmed by resolution of ST-segment elevation in the electrocardiogram.
Moreover, a sham group (n=13) was also included in the study. These animals underwent the same surgical protocol except for coronary artery occlusion.
Experimental groupsMice were randomly allocated to the following treatment groups: a) sham; b) nonreperfused MI with anti-IgG isotype antibody; c) nonreperfused MI with VEGF-A165b blocking antibody; d) reperfused MI with anti-IgG isotype antibody; and e) reperfused MI with VEGF-A165b blocking antibody. Each experimental group was composed of 13 animals, thus the final study group comprised 65 mice ().
Study in STEMI patientsThe study conformed to the Declaration of Helsinki for the use of human participants. The study protocol was approved by the local ethics committee and written informed consent was obtained from all participants.
Inclusion criteria consisted of patients with a first STEMI, treated with primary coronary intervention within 12hours of chest pain onset, and who underwent CMR imaging at 6 months post-STEMI. We prospectively enrolled 127 consecutive patients discharged between July 2013 and December 2017 with these characteristics.
Exclusion criteria comprised death (n=2), reinfarction (n=4), clinical instability (n=4) during the first 6 months postdischarge and any contraindication to CMR (n=13). Therefore, the final study group comprised 104 STEMI patients. The flowchart of patients in the study is presented in .
We recruited a control group matched in age and sex with the study group (), composed of 25 patients in whom the presence of any cardiac disease was ruled out by means of a thorough clinical history, physical examination, and echocardiographic study carried out by a clinical cardiologist.
Statistical analysisWe assessed the normality of distribution with the Kolmogorov-Smirnov test. Continuous normally distributed data are expressed as the mean±standard deviation of the mean and were compared using the unpaired Student's t test or one-way ANOVA. Nonparametric data are expressed as the median and interquartile range and were compared using the Mann-Whitney U-test. Group percentages were compared using the chi-square test or the Fisher exact test, when appropriate. Linear correlations were assessed using the Pearson correlation coefficient. Since no previous cutoff values for serum VEGF-A165b levels were validated, patients were dichotomized according to the mean value of our cohort (405 pg/mL). The association of VEGF-A165b levels (high> 405 pg/mL) with time to first MACE was determined by the Kaplan-Meier curve and the log-rank test, respectively. In serum VEGF-A165b levels in STEMI patients, 95% confidence interval (95%CI) was calculated for each group. Statistical significance was considered for 2-tailed P-values <.05. All statistical tests were performed using SPSS 19.0 (SPSS, Inc, Chicago, IL, United States).
Further details are specified in the methods section of the supplementary data.
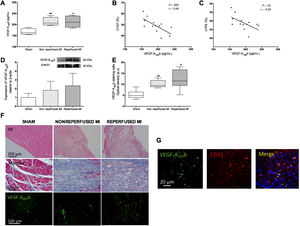
RESULTSCirculating VEGF-A165b levels were increased after MI and associated with impaired systolic functionCirculating VEGF-A165b levels were significantly augmented 21 days after MI induction in both experimental groups: nonreperfused MI (212±10 pg/mL, P <.01) and reperfused MI (213±12 pg/mL, P <.05) compared with sham (167±7 pg/mL) (figure 1A). Next, we explored the association between parameters derived from echocardiography and circulating VEGF-A165b values prior to sacrifice. An inverse correlation between VEGF-A165b serum levels with left ventricular ejection fraction (LVEF, figure 1B) and left ventricular fraction shortening (LVFS) (figure 1C) was obtained.
Serum and myocardial levels of vascular endothelial growth factor (VEGF)-A165b are increased in experimental myocardial infarction (MI) models. A: serum VEGF-A165b levels prior to sacrifice were elevated in mice undergoing MI compared with sham. Circulating VEGF-A165b levels were indirectly associated with left ventricular ejection fraction (LVEF) (B) and left ventricular fraction shortening (LVFS) (C) evaluated by transthoracic echocardiography. D: western blot analysis of VEGF-A165b protein expression in infarct myocardial samples isolated from both MI models compared with sham. E: densitometry analysis of VEGF-A165b immunofluorescence analysis. Myocardial sections were incubated with an antimouse VEGF-A165b antibody and specific labelling was visualized using Alexa Fluor 488 (VEGF-A165b, green). Images were captured and digitized and were then analyzed with Image-Pro Plus analysis software. Scoring was performed blinded on coded slides. F: representative images of hematoxylin-eosin (HE, upper panel), Masson's trichromic (central panel), and specific immunofluorescence for VEGF-A165b (lower panel, green). G: representative images showing colocalization of CD31/VEGF-A165b in infarct myocardial tissue. Immunoreactivity was visualized using Alexa Fluor 594 (CD31, red) and Alexa Fluor 488 (VEGF-A165b, green) secondary antibodies. Nuclei were stained with DAPI (blue). The correlation of VEGF-A165b with the variables derived from echocardiography (B, C) was assessed using the Pearson correlation coefficient. Continuous normally distributed data (n=13 animals per group) are expressed as mean±standard deviation and were analyzed by 1-way ANOVA analysis followed by the Bonferroni test. *P <.05, **P <.01 vs sham in A and E.
A tendency toward increased VEGF-A165b expression was detected in myocardial samples obtained from the infarcted area of both MI groups compared with sham (figure 1D). Immunofluorescence studies revealed that, although a weak constitutive expression of VEGF-A165b was observed in controls, the presence of VEGF-A165b was heightened in infarct areas from both MI groups (P <.05, figure 1D,E). Furthermore, double labelling immunofluorescence confirmed the expression of VEGF-A165b in endothelial cells (CD31+) (figure 1G).
In summary, the presence of circulating and myocardial VEGF-A165b was boosted in the 2 experimental MI models (nonreperfused and reperfused) and was associated with more depressed systolic function evaluated by echocardiography.
Blockade of VEGF-A165b activity improved angiogenesis, systolic function, and infarct size in mice undergoing reperfused MI, but not in nonreperfused MITo explore the consequences of functional VEGF-A165b blockade in vivo, we included 1 experimental group for each MI model, in which animals were treated with an intraperitoneal injection of a specific blocking anti-VEGF-A165b antibody or matched anti-IgG isotype.
Effects on systolic function and infarct sizeCompared with sham, animals undergoing either nonreperfused or reperfused MI groups and treated with anti-IgG isotype antibody displayed worse systolic function, as reflected by an increased LV end-diastolic diameter (nonreperfused: 44.4±4.1mm, reperfused: 43.8±5.7mm vs sham: 38.2±3.5mm, P <.01) and a decreased LVEF (nonreperfused: 41.4±5.8%, reperfused: 49.8±6.4% vs sham: 59.5±8.1%, P <.01) and LVFS (nonreperfused: 21.8±5.4%, reperfused: 28.8±4.5% vs sham: 35.9±6.3%, P <.01). However, no differences in systolic function were detected between nonreperfused and reperfused MI groups treated with anti-IgG isotype antibody (table 1).
Echocardiography parameters prior to sacrifice of animals in the 5 experimental groups
| Nonreperfused MI | Reperfused MI | ||||
|---|---|---|---|---|---|
| SHAM(n=13) | Anti-IgG(n=10) | Anti-VEGF-A165b(n=13) | Anti-IgG(n=12) | Anti-VEGF-A165b(n=13) | |
| LVEDD, mm | 38.2±3.5 | 44.4±4.1a | 43.5±4.8b | 43.8±5.7a | 41.9±4.6 |
| LVEF, % | 59.5±8.1 | 41.4±5.8a | 48.6±6.0a | 49.8±6.4a | 57.1±8.5c |
| LVFS, % | 35.9±6.3 | 21.8±5.4a | 26.1±5.1a | 28.8±4.5a | 34.2±6.2c |
LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; LVFS, left ventricular fraction shortening; MI, myocardial infarction; VEGF, vascular endothelial growth factor.
Data are expressed as mean±standard deviation and were analyzed by the Student t test.
Interestingly, in the reperfused MI model, treatment with a specific anti-VEGF-A165b antibody resulted in an improved LVEF (57.1±8.5% vs 49.8±6.4%, P <.05) (figure 2D,E) and LVFS (34.2±6.2% vs 28.8±4.5%, P <.05) compared with animals treated with anti-IgG antibody (figure 2D,F). In contrast, despite VEGF-A165b blockade, no improvement in systolic function was observed in either nonreperfused experimental MI groups (table 1).
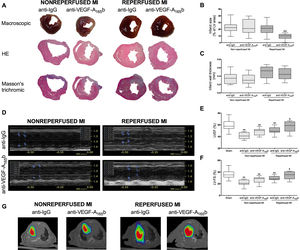
Next, macroscopic analysis of hearts isolated from the 4 experimental groups were performed (figure 2A). In mice treated with anti-IgG isotype antibody, infarct size was similar between the nonreperfused and reperfused MI groups, whereas higher infarct wall thickness and reduced LV volume was detected in animals undergoing reperfused MI compared with nonreperfused MI (table 2).
Blockade of vascular endothelial growth factor (VEGF)-A165b in reperfused, but not in nonreperfused, myocardial infarction (MI) models resulted in a reduced infarct size and enhanced systolic function. A: representative images at macroscopic (upper panel) and microscopical level stained with hematoxylin-eosin (HE, central panel), and Masson's trichromic (lower panel). In the reperfused MI model, treatment with a specific anti-VEGF-A165b antibody resulted in a reduced infarct size (B), whereas no changes in infarct wall thickness (C) were observed. Prior to sacrifice, systolic function was evaluated by echocardiography performed in a blinded manner. D: representative M-mode echocardiography images. In the reperfused MI model, treatment with a specific anti-VEGF-A165b antibody resulted in an improved (E) left ventricular ejection fraction (LVEF) and (F) left ventricular fraction shortening (LVFS), whereas treatment had no effect in the nonreperfused MI model. (G) Representative positron emission tomography/computed tomography images from the 4 MI groups. Continuous normally distributed data (n=13 mice per group) are expressed as mean±standard deviation. Data were analyzed by the unpaired Student t test. Scoring was performed by a blinded observer unaware of the experimental group. LV, left ventricle. **P <.01 vs sham group. +P <.05, ++P <.01 vs animals undergoing reperfused MI treated with anti-IgG antibody.
Analysis of cardiac structure from the 5 experimental groups
| Nonreperfused MI | Reperfused MI | ||||
|---|---|---|---|---|---|
| SHAM(n=13) | Anti-IgG(n=13) | Anti-VEGF-A165b(n=13) | Anti-IgG(n=12) | Anti-VEGF-A165b(n=13) | |
| LV area, mm2 | 0.6±0.3 | 1.0±0.5a | 1.3±0.6b | 0.7±0.2c | 0.8±0.2 |
| Infarct area, mm2 | 0.0±0.0 | 0.8±0.3 | 0.7±0.3 | 0.6±0.2 | 0.3±0.2d |
| Infarct size, % | 0.0±0.0 | 23.2±7.9 | 23.6±11.0 | 20.8±8.8 | 9.4±4.2d |
| Infarct wall thickness, mm | 0.0±0.0 | 0.4±0.1 | 0.3±0.1 | 0.5±0.1c | 0.5±0.1 |
LV, left ventricle; MI, myocardial infarction; VEGF, vascular endothelial growth factor.
Data are expressed as mean±standard deviation and were analyzed by the Student t test
Of note, treatment with a specific anti-VEGF-A165b antibody in the reperfused MI model reduced infarct size compared with treatment with anti-IgG antibody (9.4±4.2% vs 20.8±8.8%, P <.01) (figure 2B), but no changes were detected in infarct wall thickness (0.5±0.1mm vs 0.5±0.1mm) (figure 2C). In contrast, in the nonreperfused MI model, blocking the antiangiogenic VEGF-A165b isoform had no effect on infarct size or infarct wall thickness (table 2).
Last, in positron emission tomography/computed tomography images, VEGF-A165b blockade in mice undergoing reperfused MI reduced infarct size (figure 2G). Hence, not only VEGF-A165b blockade but also coronary reperfusion are necessary to improve the resultant systolic function and minimize infarct size.
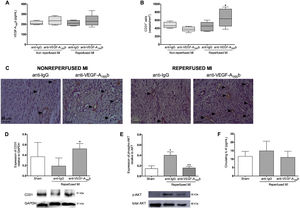
Implications of in vivo VEGF-A165b blockade on neoangiogenesisCirculating VEGF-A165b levels were similar in the 4 experimental MI models independently of the treatment received (figure 3A). In the reperfused MI models, capillary density evaluated by immunohistochemistry was significantly heightened in animals treated with anti-VEGF-A165b antibody (666±211 cells/mm2, P <.05) compared with anti-IgG (451±115 cells/mm2). However, VEGF-A165b blockade had no effect on capillary density in either group undergoing nonreperfused MI model (anti-VEGF-A165b: 377±60 cells/mm2 vs anti-IgG: 478±75 cells/mm2) (figure 3B,C). Indeed, western blotting analysis revealed increased CD31 protein expression in the infarcted myocardium isolated from reperfused MI animals treated with anti-VEGF-A165b antibody compared with anti-IgG (figure 3D).
Vascular endothelial growth factor (VEGF)-A165b blockade increased capillary density in animals undergoing reperfused, but not in nonreperfused, myocardial infarction (MI). A: serum VEGF-A165b levels prior to sacrifice were similar in all 4 experimental groups. B: quantification of CD31+ vessels. Five independent images from the infarcted area isolated from the 4 independent MI groups were taken and then analyzed by a blinded observer unaware of the experimental group. C: representative images from infarcted tissue isolated the 4 experimental groups stained with the specific vascular marker CD31. D: western blotting analysis of CD31 protein expression in myocardial tissue isolated from sham and both reperfused MI groups treated with anti-IgG or anti-VEGF-A165b blocking antibody. E: western blotting analysis of phospho AKT/total AKT in the infarct tissue isolated from sham and both reperfused MI groups treated with anti-IgG or anti-VEGF-A165b blocking antibody. F: circulating interleukin (IL)-6 levels from both reperfused MI groups treated with anti-IgG or anti-VEGF-A165b blocking antibody. Continuous normally distributed data (n=13 animals per group) are expressed as mean±standard deviation and were analyzed by the unpaired Student t test. *P <.05 vs sham. +P <.05; ++P <.01 vs animals undergoing reperfused MI treated with anti-IgG antibody.
Compared with controls, AKT phosphorylation was significantly elevated in heart samples from the reperfused MI group, whereas blockade of VEGF-A165b function reduced AKT phosphorylation (figure 3E). However, circulating interleukin-6 levels were unaltered in both reperfused MI groups compared with sham (anti-VEGF- A165b: 11.1±3.6 pg/mL, anti-IgG: 14.9±5.7 pg/mL vs sham: 11.7±3.0 pg/mL) (figure 3F)
In summary, VEGF-A165b blockade promoted neoangiogenesis, improved systolic function, and reduced infarct in the reperfused, but not in the nonreperfused MI model.
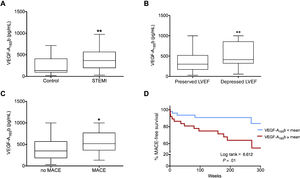
Increased serum VEGF-A165b levels in STEMI patients associated with more depressed systolic function at chronic phases and the occurrence of MACE during follow-upCharacterization of VEGF-A165b after STEMISerum VEGF-A165b levels were quantified in samples drawn 24hours after coronary revascularization from 104 STEMI patients and 25 control participants. Circulating VEGF-A165b levels were significantly elevated in STEMI patients (405±26 pg/mL, 95%CI, 355-458, P <.01) compared with controls (234±35 pg/mL, 95%CI, 161-307) (figure 4A).
Increased circulating vascular endothelial growth factor (VEGF)-A165b in ST-segment elevation myocardial infarction (STEMI) patients was associated with more depressed cardiovascular magnetic resonance (CMR)-derived LVEF at 6 months and the occurrence of adverse events during follow-up. A: serum levels of VEGF-A165b at 24hours after coronary reperfusion are boosted in STEMI patients (n=104) compared with control participants (n=23). Elevated VEGF-A165b levels are detected in STEMI patients displaying (B) depressed left ventricular ejection fraction (LVEF) at 6 months and (C) major adverse cardiac events (MACE: cardiac death, reinfarction, or readmission for heart failure) during follow-up. D: circulating VEGF-A165b and outcome Kaplan-Meier curves for MACE-free survival during follow-up depending on serum VEGF-A165b levels. Continuous normally distributed data are expressed as mean±standard error of the mean and 95% confidence interval (95% CI) and were analyzed by unpaired the Student t test. The association of VEGF-A165b levels with time to first MACE (D) were determined by the Kaplan-Meier curve and the log-rank test, respectively. *P <.05, **P <.01 vs control group.
Next, we sought to evaluate the relationship between VEGF-A165b levels at this time point and LVEF evaluated at the 6-month CMR. For this purpose, STEMI patients were dichotomized into those with depressed and those with preserved LVEF. Lower levels of circulating VEGF-A165b at 24hours after coronary reperfusion were detected in patients with preserved LVEF at the 6 month CMR (preserved: 362±32 pg/mL, 95%CI, 298-427, vs depressed: 533±44 pg/mL, 95%CI, 444-621, P <.01) (figure 4B).
Association of VEGF-A165b with the occurrence of MACEDuring follow-up (median: 186 weeks; range [33-242] weeks), there were 21 MACE (5 cardiac deaths, 9 nonfatal myocardial infarctions, and 7 readmissions for heart failure) occurred.
Patients with MACE showed augmented serum VEGF-A165b levels (552±56 pg/mL, 95%CI, 435-670, P <.05) compared with patients without MACE (413±32 pg/mL, 95%CI, 350-475) (figure 4C). Indeed, as shown in the survival curves analysis (figure 4D), VEGF-A165b levels higher than 405 pg/mL (mean) were strongly associated with an increased probability of MACE (lower: 10% vs higher: 35%, P <.01). Thus, in STEMI patients, VEGF-A165b values within the first 24hours after reperfusion were associated with a higher risk of cardiac events during follow-up.
DISCUSSIONSerum and myocardial VEGF-A165b levels were notably heightened in 2 experimental mice models (nonreperfused and reperfused MI) and were associated with more depressed systolic function evaluated by echocardiography. In vivo VEGF-A165b blockade improved capillary density, reduced infarct size, and enhanced systolic function in the reperfused, but not in the nonreperfused MI model. Last, in a cohort of STEMI patients, augmented serum VEGF-A165b concentration correlated with depressed CMR-derived LVEF in the chronic phase (6 months) after MI and with the occurrence of MACE during follow-up.
In STEMI patients, a prompt coronary reperfusion is mandatory to limit infarct size and increase patient life expectancy. Despite successful reperfusion at the epicardial level, the microvasculature is compromised in 50% to 60% of patients. Known as microvascular obstruction, this phenomenon plays a deleterious role in the resultant structural damage and the survival of STEMI patients.1,2 However, clinical and experimental studies have solidly demonstrated the spontaneous repair of microcirculation after STEMI, probably by the endogenous upregulation of proangiogenic factors soon after MI induction.7
Angiogenesis has been addressed as a potent mechanism to repair microcirculation loss post-MI, consequently producing beneficial effects on infarct healing, systolic recovery, and adequate LV remodeling. Angiogenesis consists of the development of new microvessels from preexisting capillaries in response to different signals and factors.3 Hence, understanding pathophysiological pathways that promote angiogenesis is crucial in seeking coadjuvant therapeutic alternatives, beyond prompt coronary reperfusion, to minimize the burden of MI in terms of morbidity and mortality.
Role of VEGF-A165b in mice undergoing MIVEGF-A is actively involved in tissue repair by regulating angiogenesis, vascular permeability, and inflammation. Following MI, its role has been assessed in serum and the infarcted myocardium in both patients and experimental models.4 However, alternative splicing of VEGF-A produces several mRNA isoforms, which include a distal splice-site selection in exon 8 to generate an exon 8b sequence, yielding the antiangiogenic isoform VEGF-A165b.5 In pathological angiogenic states such as diabetic retinopathy and cancer, VEGF-A165b levels are shown to decrease.8,9 In contrast, VEGF-A165b correlates with a compromised capillary density in peripheral artery disease.10,11 Particularly after MI, a previous study first reported that circulating VEGF-A165b levels rapidly peaked in the early phases (1 week) in STEMI patients, suggesting that this isoform may also contribute to the impaired neovascularization in the acute phase post-MI.6
Since no in vivo studies have so far investigated the role of VEGF-A165b post-MI, we aimed to elucidate the role of VEGF-A165b isoform in 2 experimental MI models: nonreperfused and reperfused MI.
Based on our results, circulating VEGF-A165b levels were increased in both mice models, and higher serum levels of this antiangiogenic factor were associated with lower LVEF and LVFS. Moreover, when evaluating the infarcted area from both MI models, increased expression of VEGF-A165b was detected, mainly present in the endothelial cells. Thus, the regulation of VEGF-A splicing may also be important in the context of MI in mice.
VEGF-A165b blockade as a potent target to recover capillary density and enhance systolic function after MIRegarding the previous clinical6 and experimental results, we speculate that high circulating levels of VEGF-A165b might hamper the endogenous tendency toward recovery of microvascular perfusion. In fact, when we used an in vitro coronary endothelial cell differentiation assay, VEGF-A165b blockade increased the angiogenic capacity of serum from STEMI patients.6 Indeed, a previous study in a murine model of peripheral artery disease demonstrated that treatment with a blocking antibody against VEGF-A165b isoform reversed impaired revascularization in ischemic hind limb.10,11
In the MI scenario, clinical and experimental studies have demonstrated that, although microvessel density is diminished a few minutes after coronary occlusion, massive and macroscopic microvascular obstruction appears soon after coronary reperfusion.2,7 This phenomenon is spontaneously restored due to the increased expression of proangiogenic factors immediately after ischemia onset.7 Therefore, investigating the functional blockade of VEGF-A165b in a basic model of MI could be the first step in the discovery of a promising novel therapeutic option to accelerate neoangiogenesis in the infarcted area.
Compared with the nonreperfused MI group, mice undergoing coronary reperfusion displayed an augmented infarct wall thickness, whereas LVEF and infarct size were still compromised. Timely coronary reperfusion is the main therapeutic goal to minimize irreversible apoptosis of cardiomyocytes, and consequently reduce cardiac injury. However, although early reperfusion is necessary in terms of adverse remodeling, clinical and experimental studies have reported its association with certain hemodynamic and oxidative stress damage.1,12 Therefore, discovering new coadjuvant therapies beyond reperfusion will further diminish infarct size and improve patient outcomes.
In this context, we found that blockade of VEGF-A165b activity in mice undergoing 45minutes of ischemia followed by 21 days of coronary reperfusion significantly increased capillary density, decreased infarct size, and enhanced systolic function. However, these beneficial effects were absent in the nonreperfused MI model. Based on our in vivo results, VEGF-A165b might be a promising coadjuvant therapy beyond reperfusion to further diminish infarct size and microvascular damage.
To understand the mechanistic role of VEGF-A165b, we evaluated AKT expression due to its implication in multiple signaling pathways in the regulation of angiogenesis mediated by VEGF-A.13 Our present data suggest that the AKT signaling pathway is activated post-MI and that the antiangiogenic effect of VEGF-A165b could be partially caused by AKT activation.
VEGF-A165b has been reported to reduce tumor necrosis factor-α in retinal pigmented epithelial cells, thus exerting anti-inflammatory effects.9 However, according to our results, serum interleukin-6 levels in both reperfused MI groups at day 21 post-MI were similar to those in controls, probably due to the almost complete resolution of the proinflammatory milieu occurring during the first few days after MI induction.
In conclusion, VEGF-A165b may represent a new coadjuvant pharmacological target to coronary reperfusion (the current gold standard therapy for STEMI patients), since its blockade might boost the activities of proangiogenic growth factors released at the very beginning of ischemia.7
Systolic and prognostic implications of VEGF-A165b in STEMI patientsImpaired microcirculation in STEMI patients correlates with adverse ventricular remodeling and the occurrence of cardiac events during follow-up.1,2 Therefore, understanding the role of a novel potent regulator of angiogenesis in this scenario might be crucial.
As noted previously in a preliminary cohort of 50 patients,6 and confirmed here, circulating VEGF-A165b levels are notably elevated in STEMI patients compared with healthy patients. Indeed, higher messenger ribonucleic acid (mRNA) expression of this antiangiogenic factor has been demonstrated in peripheral blood from patients with coronary heart disease.14 Although elevated VEGF-A165b values have already been reported to correlate with compromised systolic function in the acute phase (1 week post-MI), the novelty of this study lies in associating circulating VEGF-A165b values with the resultant systolic function in the chronic phase (6 months post-MI) and patient prognosis in the largest cohort of STEMI patients so far. Based on our results, higher serum VEGF-A165b levels are associated with more depressed CMR-derived LV function in the chronic phase (6 months) post-MI and the occurrence of MACE during follow-up. Harada et al.15 also noted the role of this isoform in patient prognosis in a very small cohort of STEMI patients.
Our specific objective was to establish an association between this novel factor and LV function, both in the acute6 and chronic phases, as well as with patient prognosis. In this study, we did not intend to demonstrate its independent value for risk stratification of STEMI patients beyond the current armamentarium already available for this goal.
In summary, and regarding the clinical and experimental results, higher VEGF-A165b levels were associated with worse systolic function and patient prognosis. This is probably due to its interference in the endogenous physiological reestablishment of the compromised microcirculation driven by the upregulation of proangiogenic molecules in the infarct region.7 Hence VEGF-A165b blockade might represent a promising therapeutic strategy to improve angiogenesis after STEMI, and consequently to increase systolic function and patient life expectancy.
Study limitationsDue to the small number of patients, this is only a proof of concept study to explore a marker in an animal model and in a limited number of patients. Further validation is required in a larger cohort of STEMI patients.
CONCLUSIONSWe provide evidence that endogenous VEFG-A165b is significantly elevated in experimental animal models of MI and in STEMI patients. Its in vivo neutralization promotes angiogenesis, reduces infarct size, and enhances systolic function as long as coronary reperfusion is allowed. In STEMI patients, increased concentrations of this isoform are correlated with worse systolic function and a higher incidence of adverse events during follow-up. Pharmacological modulation of VEGF-A165b might represent a potential novel target as a coadjuvant therapy to coronary reperfusion and merits further clinical research.
FUNDINGThis work was supported by Instituto de Salud Carlos III and Fondos Europeos de Desarrollo Regional FEDER (research grants PIE15/00013, PI17/01836, PI18/00209, and CIBERCV16/11/00486 and a postgraduate contract FI18/00320 to C. R.-N. and FI19/00033 to L. H.); the Spanish Ministry of Economy and Competiveness (SAF2017-89714-R); by Sociedad Española de Cardiología (SEC2017 grant); and by Generalitat Valenciana (GV/2018/116, CDEI-04/20 and AICO/2019/250).
CONFLICTS OF INTERESTNothing to declare.
- -
Despite complete revascularization, the myocardial microvasculature is impaired in approximately 50% of MI patients. Discovering coadjuvant therapies to diminish microvascular damage is essential. Angiogenesis helps to reestablish compromised microvasculature post-MI. We aimed to assess the role of the antiangiogenic VEGF-A165b isoform.
- -
We demonstrate that experimental MI models displayed elevated circulating and myocardial VEGF-A165b levels. Its in vivo blockade increased neoangiogenesis, reduced infarct size, and enhanced systolic function in reperfused, but not in nonreperfused, MI models. In patients, higher VEGF-A165b values correlated with depressed ejection fraction and worse outcomes. Blocking this antiangiogenic factor may exert beneficial effects post-MI.
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.rec.2020.03.013