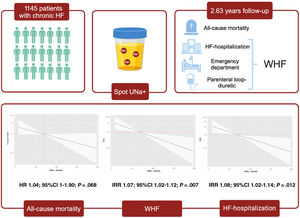
Spot determination of urinary sodium (UNa+) has emerged as a useful tool for monitoring diuretic response in patients with acute heart failure (AHF). However, the evidence in outpatients is scarce. We aimed to examine the relationship between spot UNa+ levels and the risk of mortality and worsening heart failure (WHF) events in individuals with chronic HF.
MethodsThis observational and ambispective study included 1145 outpatients with chronic HF followed in a single center specialized HF clinic. UNa+ assessment was carried out 1-5 days before each visit. The endpoints of the study were the association between UNa+ and risk of a) long-term death and b) AHF-hospitalization and total WHF events (including AHF-hospitalization, emergency department visits or parenteral loop-diuretic administration in HF clinic), assessed by multivariate Cox and negative binomial regressions.
ResultsThe mean±standard deviation of age was 73±11 years, 670 (58.5%) were men, 902 (78.8%) were on stable NYHA class II, and 595 (52%) had LFEF ≥50%. The median (interquartile range) UNa+ was 72 (51-94) mmol/L. Over a median follow-up of 2.63 (1.70-3.36) years, there were 293 (25.6%) deaths and 382 WHF events (244 AHF-admissions) in 233 (20.3%) patients. After multivariate adjustment, baseline UNa+ was inverse and linearly associated with the risk of total WHF (IRR, 1.07; 95%CI, 1.02-1.12; P=.007) and AHF-admissions (IRR, 1.08; 95%CI, 1.02-1.14; P=.012) and borderline associated with all-cause mortality (HR, 1.04; 95%CI, 0.99-1.09; P=.068).
ConclusionsIn outpatients with chronic HF, lower UNa+ was associated with a higher risk of recurrent WHF events.
Keywords
Abreviations
Congestion plays a fundamental role in heart failure (HF) pathophysiology1 with approximately 80% of HF decompensations attributed to either the onset or worsening congestion.2,3 Volume overload is mainly passive and secondary to sodium retention.4,5 Thereby, urinary sodium (UNa+) has become a useful tool for assessing diuretic response and identifying patients with diuretic resistance.6,7 24-hour UNa+ is the standard method for quantifying natriuresis.8 However, its use in clinical practice is cumbersome, especially for follow-up.8
In recent years, the spot determination of UNa+ has emerged as a valid alternative to predict and monitor diuretic response in patients with acute HF (AHF) and a reliable prognostic biomarker.8 However, the evidence in an outpatient setting is scarce, and there are no prior studies that have evaluated the clinical utility of risk stratification for predicting long-term outcomes, especially HF morbidity burden in chronic HF.9,10
The objective of this work was to evaluate the association of spot UNa+ and the risk of long-term mortality and total worsening heart failure (WHF) events in outpatients with chronic HF.
METHODSStudy populationThis is an observational, single-center, and ambispective study in which 1145 consecutive outpatients with stage C chronic ambulatory HF were included. We enrolled patients along the entire spectrum of functional class. Patients with a hospitalization within the prior 30 days were excluded. All patients in the sample were routinely followed up in a single center specialized ambulatory HF clinic in Spain from October 2016 to October 2021. The diagnosis of HF was made according to the current guidelines as the presence of symptoms and/or signs with left ventricular ejection fraction (LVEF) <50% or evidence of cardiac structural and/or functional abnormalities.11,12 Pre-established electronic questionnaires were used to record all the information related to demographic data, complete previous medical history, vital signs, physical examination, 12-lead electrocardiogram, echocardiography, laboratory tests, and pharmacological therapies in each medical visit.
The study was designed conformed to the principles outlined in the Declaration of Helsinki and approved by the institutional local review ethical committee (Comité Ético de Investigación Clínica, Hospital Clínico Universitario de Valencia).
Urine sampling, storage, and analysisPatients were instructed to collect a first void morning urine sample. Patients who were on chronic oral diuretic therapy were instructed to take the diuretic only after collection of first morning void. Urine was collected in a disposable urine collection cup. Afterward, a spot sample was aspirated from the sealed collection cup by using the aspiration port and immediately placed the labeled vacuum tube in their freezer. All urine samples were collected 1-5 days before each scheduled visit and the determination of UNa+ was carried out using ion selective electrode indirect potentiometry. During the study period, no specific therapeutic recommendations were based on UNa+.
At the same time, a blood sample was taken, including renal function parameters, plasma sodium, potassium, hemoglobin, NTproBNP, and CA125 measured using commercially available immunoassay kits (Elecsys NT-proBNP assay and Elecsys CA125 II assay, Roche Diagnostics, Germany).
Endpoints and follow upThe endpoints of interest were long-term all-cause death and the number of episodes of WHF (including AHF hospitalizations, urgent visits to the Emergency Department, and parenteral loop diuretic administration in the HF clinic). Cardiovascular mortality, defined as death due to diseases of the heart or blood vessels (most commonly heart failure, coronary disease, sudden cardiac death, or stroke) was also explored. The assessment of outcomes was performed by verifying the patient's survival status or occurrence of readmission by reviewing electronic medical records of the regional public health care system. This assessment used data from the SIA-GAIA and Orion Clinic electronic databases, which comprehensively record all medical interactions occurring in the public healthcare system of the Valencian Community.
The median number of follow-up visits in the HF unit per year was 3 (2-4), and survival status at 6 months, 1 year, and 2 years was available in 98.1%, 94.3%, and 75.0% of the sample, respectively.
Statistical analysisContinuous variables are expressed as mean±standard deviation or median (interquartile range) when appropriate. Discrete variables were summarized as percentages. Baseline UNa+ was evaluated as a continuum and stratified in quartiles for analysis.
Comparisons across UNa+ quartiles categories were performed by Chi squared test for categorical variables and for continuous variables 1-way analysis of variance (ANOVA) or Kruskal–Wallis tests as appropriate. The cumulative probability of all-cause mortality across UNa+ quartiles was estimated by the Kaplan-Meier method, and differences compared by the log-rank test. The association between the exposure and mortality was assessed by multivariate Cox proportional hazard regression model. Estimates of risk were reported as hazard ratios (HR). For cardiovascular mortality, we used a Cox regression analysis adapted for non-cardiovascular mortality as a competing event. For examining the association between UNa+ and total WHF events and AHF-hospitalizations during follow-up, we performed a multivariate negative binomial regression model that simultaneously models the number of WHF (as counts) or AHF-hospitalizations and all-cause mortality (as a terminal event). Regression estimates for both outcomes were mutually adjusted by means of shared frailty (accounting for the positive correlation between the two outcomes)13. Risk estimates for WHF and AHF-hospitalizations were expressed as incidence rate ratios (IRR).
The linearity assumption for all continuous variables was simultaneously tested, and the variable transformed, if appropriate, with fractional polynomials. Covariates included in the final multivariate models for all the endpoints were based on biological plausibility and were: age, sex, previous admission for AHF, history of hypertension, history of ischemic heart disease, functional class evaluated by the NYHA scale, Charlson's comorbidity index, heart rate, systolic blood pressure, glomerular filtration rate by CKD-EPI equation, blood urea nitrogen/creatinine ratio, serum sodium, NT-proBNP, CA125, furosemide equivalent dose and treatment with renin-angiotensin-aldosterone system inhibitors and beta-blockers.
A 2-sided p-value of <.05 was the threshold used for significance in all analyses. The analysis was implemented with the Merlin package within STATA 18.1 (Stata Corp., United States).
RESULTSThe mean±standard deviation of age was 73±11 years, 670 (58.5%) patients were male, 902 (78.8%) were on stable NYHA class II, and 595 (52%) had LVEF ≥ 50%. The median (interquartile range) UNa+ at first visit was 72 (51-94) mmol/L.
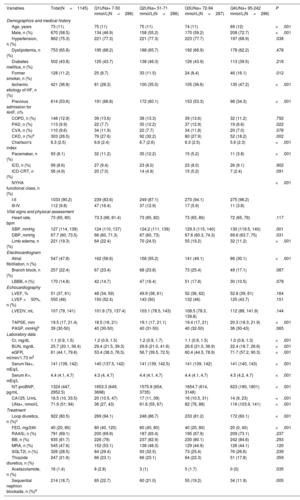
Table 1 shows the baseline characteristics of the overall cohort based on UNa+ quartiles (Q1=7-50 mmol/L; Q2=51-71 mmol/L; Q3=72-94 mmol/L; Q4=92-242 mmol/L). Patients in the lowest UNa+ quartile showed a worse baseline risk profile. They were older and with greater comorbidity burden (higher Charlson's index), with more previous admissions for AHF, worse NYHA functional class, lower blood pressure, and a higher rate of peripheral edema. These patients also displayed lower glomerular filtration rate and higher levels of NT-proBNP and CA125 and were more frequently on loop diuretics and with higher furosemide equivalent doses as well as sequential nephron blockade (table 1). Regarding echocardiographic parameters, we did not find significant differences in LVEF. However, those patients with lower UNa+ showed lower tricuspid annulus plane systolic excursion.
Baseline characteristics by UNa+ quartiles
| Variables | Total(N=1145) | Q1UNa+ 7-50 mmol/L(N=286) | Q2UNa+ 51-71 mmol/L(N=286) | Q3UNa+ 72-94 mmol/L(N=287) | Q4UNa+ 95-242 mmol/L(N=286) | P |
|---|---|---|---|---|---|---|
| Demographics and medical history | ||||||
| Age, years | 73 (11) | 75 (11) | 75 (11) | 74 (11) | 69 (12) | <.001 |
| Male, n (%) | 670 (58.5) | 134 (46.9) | 158 (55.2) | 170 (59.2) | 208 (72.7) | <.001 |
| Hypertension, n (%) | 862 (75.3) | 221 (77.3) | 221 (77.3) | 223 (77.7) | 197 (68.9) | .038 |
| Dyslipidemia, n (%) | 753 (65.8) | 195 (68.2) | 188 (65.7) | 192 (66.9) | 178 (62.2) | .478 |
| Diabetes mellitus, n (%) | 502 (43.8) | 125 (43.7) | 138 (48.3) | 126 (43.9) | 113 (39.5) | .218 |
| Former smoker, n (%) | 128 (11.2) | 25 (8.7) | 33 (11.5) | 24 (8.4) | 46 (16.1) | .012 |
| Ischemic etiology of HF, n (%) | 421 (36.8) | 81 (28.3) | 100 (35.0) | 105 (36.6) | 135 (47.2) | <.001 |
| Previous admission for AHF, n% | 614 (53.6) | 191 (66.8) | 172 (60.1) | 153 (53.3) | 98 (34.3) | <.001 |
| COPD, n (%) | 148 (12.9) | 39 (13.6) | 38 (13.3) | 39 (13.6) | 32 (11.2) | .792 |
| PAD, n (%) | 113 (9.9) | 22 (7.7) | 35 (12.2) | 37 (12.9) | 19 (6.6) | .022 |
| CVA, n (%) | 110 (9.6) | 34 (11.9) | 22 (7.7) | 34 (11.8) | 20 (7.0) | .078 |
| CKD, n (%)a | 303 (26.5) | 79 (27.6) | 92 (32.2) | 80 (27.9) | 52 (18.2) | .002 |
| Charlson's index | 6.3 (2.5) | 6.6 (2.4) | 6.7 (2.6) | 6.3 (2.5) | 5.6 (2.3) | <.001 |
| Pacemaker, n (%) | 93 (8.1) | 32 (11.2) | 35 (12.2) | 15 (5.2) | 11 (3.8) | <.001 |
| ICD, n (%) | 99 (8.6) | 27 (9.4) | 23 (8.0) | 23 (8.0) | 26 (9.1) | .902 |
| ICD-CRT, n (%) | 56 (4.9) | 20 (7.0) | 14 (4.9) | 15 (5.2) | 7 (2.4) | .091 |
| NYHA functional class, n (%) | <.001 | |||||
| I-II | 1033 (90.2) | 239 (83.6) | 249 (87.1) | 270 (94.1) | 275 (96.2) | |
| III-IV | 112 (9.8) | 47 (16.4) | 37 (12.9) | 17 (5.9) | 11 (3.8) | |
| Vital signs and physical assessment | ||||||
| Heart rate, bpm | 73 (65, 80) | 73.3 (66, 81.4) | 73 (65, 82) | 73 (65, 80) | 72 (65, 78) | .117 |
| SBP, mmHg | 127 (114, 139) | 124 (110, 137) | 124.2 (111, 139) | 129.3 (115, 140) | 130 (119.5, 140) | .001 |
| DBP, mmHg | 67.7 (60, 73.5) | 66 (60, 71.3) | 67 (60, 73) | 67.6 (60.3, 74.3) | 69.6 (63.7, 75) | .031 |
| Limb edema, n (%) | 221 (19.3) | 64 (22.4) | 70 (24.5) | 55 (19.2) | 32 (11.2) | <.001 |
| Electrocardiogram | ||||||
| Atrial fibrillation, n (%) | 547 (47.8) | 162 (56.6) | 158 (55.2) | 141 (49.1) | 86 (30.1) | <.001 |
| Branch block, n (%) | 257 (22.4) | 67 (23.4) | 68 (23.8) | 73 (25.4) | 49 (17.1) | .087 |
| LBBB, n (%) | 170 (14.8) | 42 (14.7) | 47 (16.4) | 51 (17.8) | 30 (10.5) | .078 |
| Echocardiography | ||||||
| LVEF, % | 51 (37, 61) | 48 (34, 59) | 49.9 (38, 61) | 52 (36, 62) | 52.8 (39, 61) | .164 |
| LVEF <50%, n (%) | 550 (48) | 150 (52.4) | 143 (50) | 132 (46) | 125 (43.7) | .151 |
| LVEDV, mL | 107 (79, 141) | 101.8 (73, 137.4) | 103.1 (78.5, 143) | 108.5 (78.3, 139.8) | 112 (88, 141.9) | .144 |
| TAPSE, mm | 19.5 (17, 21.4) | 18.5 (16, 21) | 19.1 (17, 21.1) | 19.4 (17, 21) | 20.3 (18.3, 21.9) | <.001 |
| PASP, mmHgb | 39 (30-50) | 40 (30-50) | 40 (31-50) | 40 (32-50) | 36 (30-43) | .065 |
| Laboratory data | ||||||
| Cr, mg/dL | 1.1 (0.9, 1.5) | 1.2 (0.9, 1.5) | 1.2 (0.9, 1.7) | 1.1 (0.9, 1.5) | 1.0 (0.8, 1.3) | <.001 |
| BUN, mg/dL | 25.7 (20.1, 36.4) | 29.4 (21.5, 39.3) | 29.6 (21.0, 41.6) | 26.6 (21.0, 36.9) | 22.4 (18.7, 26.6) | <.001 |
| eGFR, ml/min/1.73 m2 | 61 (44.1, 79.6) | 53.4 (38.3, 76.5) | 56.7 (39.5, 72.5) | 60.4 (44.5, 78.9) | 71.7 (57.2, 90.3) | <.001 |
| Serum Na+, mEq/L | 141 (139, 142) | 140 (137.5, 142) | 141 (139, 142.5) | 141 (139, 142) | 141 (140, 143) | <.001 |
| Serum K+, mEq/L | 4.4 (4.1, 4.7) | 4.3 (4, 4.7) | 4.4 (4.1, 4.7) | 4.4 (4.1, 4.7) | 4.5 (4.2, 4.7) | <.001 |
| NT-proBNP, pg/mL | 1324 (447, 2952.5) | 1653.3 (649, 3698) | 1575.9 (654, 3735) | 1654.7 (614, 3148) | 623 (190, 1801) | <.001 |
| CA125, U/mL | 16.5 (10, 33.5) | 20 (10.5, 47) | 17 (11, 39) | 16 (10.5, 31) | 14 (9, 23) | <.001 |
| UNa+, mmol/L | 71.9 (51; 94) | 36 (27, 43) | 61.6 (55, 67) | 82 (76, 88) | 116 (103.9, 141) | <.001 |
| Treatment | ||||||
| Loop diuretics, n (%)c | 922 (80.5) | 269 (94.1) | 248 (86.7) | 233 (81.2) | 172 (60.1) | <.001 |
| FED, mg/24h | 40 (20, 80) | 80 (40, 120) | 60 (40, 80) | 40 (20, 80) | 20 (0, 40) | <.001 |
| RAASi, n (%) | 791 (69.1) | 200 (69.9) | 187 (65.4) | 195 (67.9) | 209 (73.1) | .237 |
| BB, n (%) | 935 (81.7) | 226 (79) | 237 (82.9) | 230 (80.1) | 242 (84.6) | .293 |
| MRA, n (%) | 545 (47.6) | 152 (53.1) | 138 (48.3) | 129 (44.9) | 126 (44.1) | .120 |
| SGLT2i, n (%) | 326 (28.5) | 84 (29.4) | 93 (32.5) | 73 (25.4) | 76 (26.6) | .239 |
| Thiazide diuretics, n (%) | 247 (21.6) | 66 (23.1) | 66 (23.1) | 64 (22.3) | 51 (17.8) | .359 |
| Acetazolamide, n (%) | 16 (1.4) | 8 (2.8) | 3 (1) | 5 (1.7) | 0 (0) | .035 |
| Sequential nephron blockade, n (%)d | 214 (18.7) | 65 (22.7) | 60 (21.0) | 55 (19.2) | 34 (11.9) | .005 |
Values are expressed as mean (SD) and median (percentile 25% to percentile 75%). Categorical variables are presented as percentages. AHF, acute heart failure; CA125, antigen carbohydrate 125; CKD, chronic kidney disease, COPD, chronic pulmonary obstructive disease; CRT, cardiac resynchronization therapy; CVA, cerebrovascular accident DBP, dyastolic blood pressure; eGFR, estimated glomerular filtration rate assessed by CKD-EPI equation; FED, furosemide equivalent dose; HF, heart failure; ICD, implantable cardioverter defibrillator; K+, potassium; LBBB, left bundle branch block; LVEDV, left ventricular end-diastole volume; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; Na+, sodium; NT-proBNP, N-terminal propeptide brain natriuretic peptide; NYHA, New York Heart Association; PAD, peripheral artery disease; RAASi, renin.-angiotensin-aldosterone-inhibitors; SBP, systolic blood pressure; SGLT2i, sodium–glucose cotransporter 2 inhibitors; PASP, pulmonary artery systolic pressure; TAPSE, tricuspid annulus plane systolic excursion; UCl+, urinary chloride; UCr, urinary creatinine; UK+, urinary potassium; UNa+, urinary sodium.
At a median (p25% to p75%) follow-up of 2.63 (1.70-3.36) years, we registered 293 (25.6%) deaths, 382 episodes of WHF, which occurred in 233 patients (20.3%), and 244 AHF-admissions in 170 patients (14.85%). The causes of death were cardiovascular and non-cardiovascular in 147 (12.8%) and 146 (12.7%) cases. The most common cardiovascular death cause was HF (61 patients, 5.3% of the sample). A substantial number of patients presented with recurrent WHF events as there were 62, 18, 8, 5 and 1 patients with 2, 3, 4, 5 and 8 events, respectively.
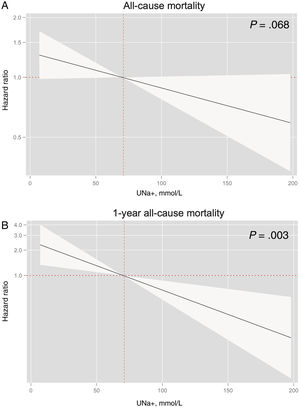
Baseline urinary sodium and risk of mortalityKaplan-Meier plots showed higher risk of mortality in the lower UNa+ quartiles (figure 1). These differences were mainly found during the first year of follow-up, with a posterior overlap between Q1 and Q2 after this period (figure 1). After multivariate adjustment, UNa+ was no longer associated with a significant risk of long-term mortality, however, differences were borderline significant (HR per 10 mmol/L decrease in UNa+, 1.04; 95%CI, 0.99-1.09; P=.068) (figure 2A).
In a sensitivity analysis, limiting the follow-up to 1-year, lower UNa+ was significantly and inversely associated with the risk of all-cause mortality despite multivariate adjustment (HR per 10 mmol/L decrease in UNa+, 1.14; 95%CI, 1.05-1.25; P=.003) (figure 2B). Compared with the highest quartile, patients belonging to the lowest UNa+ quartile showed more than 2-fold increase of risk (HR, 2.21; 95%CI, 1.03-4.73; P=.041) (table 2). Patients in Q2 and Q3 did not show a significant increased risk (HR, 1.04; 95%CI, 0.48-2.27; P=.915 and HR, 0.87; 95%CI, 0.38-2.03; P=.754; respectively).
Risk estimates for the risk of mortality, heart failure hospitalization and worsening heart failure
| Events | HR (95%CI) | P |
|---|---|---|
| All-cause mortality | ||
| Q4 (reference) | ||
| Q1 | 1.23 (0.83-1.83) | .303 |
| Q2 | 1.09 (0.74-1.60) | .661 |
| Q3 | 0.88 (0.59-1.31) | .525 |
| 1-year all-cause mortality | ||
| Q4 (reference) | ||
| Q1 | 2.21 (1.03-4.73) | .041 |
| Q2 | 1.04 (0.48-2.27) | .915 |
| Q3 | 0.87 (0.38-2.03) | .754 |
| WHF | ||
| Q4 (reference) | ||
| Q1 | 1.70 (1.11-2.61) | .015 |
| Q2 | 1.34 (0.88-2.03) | .177 |
| Q3 | 1.00 (0.66-1.53) | .994 |
| HF-hospitalizations | ||
| Q4 (reference) | ||
| Q1 | 1.83 (1.10-3.04) | .020 |
| Q2 | 1.43 (0.87-2.35) | .160 |
| Q3 | 1.05 (0.63-1.75) | .839 |
Risk estimates for the risk of mortality, HF-hospitalization and WHF across UNa+ quartiles in the multivariable models. 95%CI, 95% confidence interval; HF, heart failure; IRR, incidence rate ratio; Q, quartile; UNa+, urinary sodium; WHF, worsening heart failure.
Regarding cardiovascular mortality, after multivariate adjustment, UNa+ was also not significantly associated with mortality (HR per 10 mmol/L decrease in UNa+, 1.05; 95%CI, 0.99-1.11; P=.085 (figure 1 of the supplementary data).
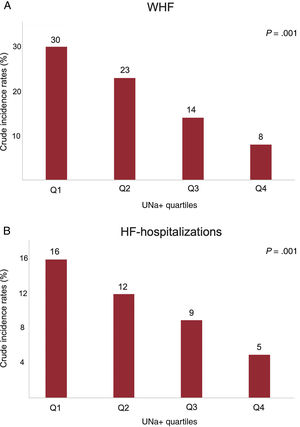
Baseline urinary sodium and total worsening heart failure eventsThe rates of WHF (per 100 person-years) significantly increased from higher to lower UNa+ quartiles, as shown in figure 3A. The same was true when only AHF-hospitalizations were considered (figure 3B).
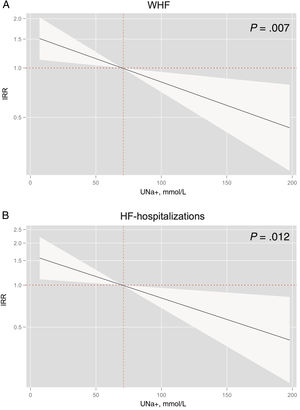
After multivariate adjustment, UNa+ remained significantly and inversely associated with the risk of total WHF episodes, including AHF-hospitalization (figure 4). For every 10 mmol/L decrease in UNa+, the risk of WHF and AHF-hospitalizations increased by 1.07 (95%CI, 1.02-1.12; P=.007) and 1.08 (95%CI, 1.02-1.14; P=.012), respectively (figure 3A,B). Compared to patients in the upper quartile, patients in the lowest quartile (≤ 50 mmo/L) had a significantly higher risk of episodes of WHF (IRR, 1.70; 95%CI, 1.11-2.61; P=.015), also including higher risk of AHF-readmission (IRR, 1.83; 95%CI, 1.10-3.04; P=.020) (table 2).
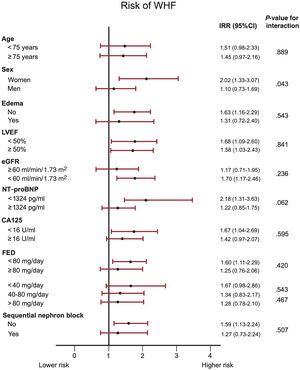
Overall, under the same multivariate scenario, we did not find evidence of heterogeneity between UNa+ ≤ 50 mmol/L and risk of WHF among the most relevant characteristics of the population including age < or ≥ 75 years, presence or absence of edema, LVEF <or ≥ 50%, estimated glomerular filtration rate < or ≥ 60ml/min/1.73m2, NTproBNP and CA125 below or over the median, furosemide equivalent dose or sequential nephron block (figure 5). We did find a signal of a greater association of UNa+ ≤ 50 mmol/L in women vs men (P for interaction=.043) for predicting WHF (figure 5).
Subgroup analysis among urinary sodium (UNa+) ≤ 50 mmol/L versus UNa+ >50 mmol/L based on risk of worsening heart failure (WHF) events.
CA125: antigen carbohydrate 125, CI: confidence interval; eGFR; estimated glomerular filtration rate assessed by CKD-EPI equation; FED: furosemide equivalent dose; IRR: incidence rate ratio; LVEF: left ventricle ejection fraction; NT-proBNP; N-terminal propeptide brain natriuretic peptide.
A sensitivity analysis exploring the independent association between UNa+ and 1-year time to WHF showed that lower UNa+ was also associated with the risk of this endpoint (HR per 10 mmol/L decrease in UNa+, 1.13; 95%CI, 1.03-1.24; P=.010) as shown in figures 2 and 3 of the supplementary data). Figure 6 summarizes the main methods and the results of the work.
DISCUSSIONIn this observational study, which includes a comprehensive cohort of outpatients with chronic HF, lower spot UNa+ identified a higher risk of long-term recurrent episodes of WHF. Specifically, spot UNa+ ≤50 mmol/L identified a subset of patients at higher risk of WHF episodes, including AHF hospitalizations. The association between lower UNa+ and the risk of mortality was not significant at long-term follow-up. This phenomenon could potentially stem from the extended duration of study follow-up, given that UNa+ exhibits considerable variability and may more accurately reflect short-term mortality risk. Notably, our assessment of its correlation with all-cause mortality within one year revealed a significantly elevated risk of death among patients in the lowest quartile.
While much of the existing literature on UNa+ pertains to its relevance in acute HF exacerbations, we contend that this biomarker also holds prognostic significance in the outpatient setting. To the best of our knowledge, this study includes the largest cohort evaluating the use of spot UNa+ levels among a wide spectrum of ambulatory HF. The association between lower UNa+ and the burden of HF morbidity was consistent among more representative subgroups.
Predicting worsening heart failure: an unmet challengeThe prediction of HF decompensations, including both HF-related hospitalizations and WHF without hospital admission, remains a clinical challenge.14 While factors linked to mortality risk are well-established, with several extensively validated scoring systems, the prediction of HF hospitalizations or WHF episodes remains inadequately addressed.15,16 Indeed, there is a paucity of scoring systems specifically designed for predicting HF decompensations, and those that do exist demonstrate low discriminatory accuracy.17,18 Furthermore, many existing models focus on predicting the initial HF event, disregarding the fact that most patients experience recurrent events, and they fail to adjust for mortality as a terminal and competing event.19 In this context, the present findings reinforce the consistent utility of spot urinary sodium levels in predicting the burden of WHF.
Spot urinary sodium for risk stratification: prior studiesNumerous contemporary studies have explored the prognostic utility of spot UNa+ in AHF exacerbations.20–30 These studies are small, mainly observational, and heterogeneous regarding the cutoff values and the timing of the measure. Nevertheless, they seem to agree that a lower UNa+ is related to worse diuretic response and higher risk of adverse vents both during the decompensation and in the long term.20–30 Consequently, current clinical guidelines and expert recommendations advocate for the incorporation of UNa+ assessment in diuretic management protocols during the initial 24hours of HF decompensation.12,31
The 2 largest studies conducted to date include the recently published ENACT-HF32 involving 401 patients and the PUSH-AHF trial33 comprising 310 patients, with the latter being the only randomized trial. These interventional studies, comparing diuretic regimens guided by standardized UNa+-based protocols versus local practices, demonstrate higher 24-hour UNa+ excretion and increased natriuresis and diuresis in the intervention groups, attributed to more aggressive up-titration of loop diuretics and augmented use of adjunctive diuretic agents.32,33
Spot urinary sodium in chronic heart failureThe evidence is much scarcer in the ambulatory HF. We have not identified previous studies regarding one single spot UNa+ measure as a marker of long-term prognosis. There are, however, preliminary studies that encourage us to think about the usefulness of urinary sodium also in outpatients. Elias et al. performed a combined analysis in 263 optimized HF patients based on the concentration of UNa+ in an isolated urine sample (with a cut-off point of 80 mEq/L) and the daily dose of furosemide (cutoff point of 80mg/day). They found that patients with UNa+ < 80 mEq/L and furosemide >80mg/day had higher 5-year mortality rates.34 Moreover, the role of UNa+ for monitoring the course of the disease is unknown. A very interesting study is that of Martens et al. which already suggests NaU+ as a telemonitoring tool for outpatients with HF. This single-center observational trial prospectively followed 80 patients who collected first-void urine once a week for 30 consecutive weeks. A drop in urinary sodium concentration was observed in the week before an event, which returned to initial values after decongestion.35
The current findings support the routine measurement of spot NaU+ for risk stratification in ambulatory HF setting. Interestingly, the association between lower NaU+ and WHF burden was homogenous among more prevalent subgroups including those with preserved LVEF or renal dysfunction. Likewise, and consistent with AHF studies, a threshold of 50 mmol/L emerges as a useful cutoff for use in clinical practice.
Clinical implications and future directionsFirst, and according to the present findings, we advocate measuring NaU+ for estimating the WHF burden of ambulatory patients with HF. With the current data we cannot unravel the exact pathophysiological mechanism of lower UNa+; however, we speculate lower UNa+ identifies a subset of patients with more advanced disease with greater neurohormonal activation, higher sodium tubular reabsorption, and greater diuretic resistance. Further studies are warranted to better define the crucial determinants of lower UNa+ and their clinical implications along the full spectrum of HF patients. NaU+ trajectory and whether serial assessment may reveal changes in risk and might be used for monitoring or guiding decongestion strategies in the ambulatory setting remains also unknown.
Study limitationsWe acknowledge several limitations. First, this is an observational and single-center study in which the retrospective collection of the data and the number of unmeasured confounders may be playing a role. Second, the UNa+ value was not blind to the clinician in charge of the patient. Despite there being no specific clinical recommendation based on an isolated determination of UNa+ in the outpatient setting, we cannot rule out it may occur in some cases. Third, longitudinal assessment of UNa+ and changes in treatment were not evaluated. Fourth, with the current design, we could not better dissect the link between hemodynamic status-UNa+ and prognosis. Fifth, renal outcomes during follow-up were not assessed. Sixth, how time from prior hospitalization or intravenous loop diuretic administration and UNa+ measurement to ambulatory visit may influence these findings cannot be elucidated in this study. Finally, we did not register echographic parameters of fluid overload.
CONCLUSIONSIn patients with chronic stable HF, lower baseline spot UNa+ was associated with increased risk WHF events at long-term follow-up, including AHF-hospitalizations.
- -
Spot UNa+ is a useful prognostic biomarker in AHF and can overcome 24-hour urine sodium measurement.
- -
A diuretic therapy guided by UNa+ seems to increase natriuresis and diuresis at short term after a hospitalization for AHF.
- -
UNa+ is a valid parameter for identify outpatients with chronic HF in higher risk of recurrent episodes of WHF, including HF hospitalizations.
- -
Lower spot UNa+ was related with increased risk of 1 year mortality, and borderline related to long-term risk of all-cause mortality.
- -
UNa+ ≤ 50 mmol/L compared to a higher level implies a situation of increased risk of WHF irrespective of age, glomerular filtration rate, LVEF, diuretic scheme, and natriuretic peptides.
This work was supported in part by grants from Instituto de Salud Carlos III (grant FIS PI20/00392) and CIBER Cardiovascular [grant numbers 16/11/00403 and 16/11/00420].
ETHICAL CONSIDERATIONSThe study was designed conformed to the principles outlined in the Declaration of Helsinki and approved by the ethics committee of Hospital Clínico Universitario, Valencia. As an observational study with a retrospective event analysis in which a percentage of patients were deceased at the time of data collection, the ethics committee accepted the absence of informed consent in this study. SAGER guidelines regarding potential sex/gender biases were followed.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCENo use of artificial intelligence was made in the writing of this manuscript.
AUTHORS’ CONTRIBUTIONSM. Lorenzo participated in data collection, writing the draft of the manuscript, and preparing tables and figures. R. de la Espriella, G. Miñana, E. Santas and S. Villar have participated in the follow-up of the patients and reviewed the manuscript. G. Núñez participated in the follow-up of the patients and data collection. A. Carratalá and E. Rodríguez have carried out the measurement of the laboratory samples and have reviewed the final version of the manuscript. N. Valls has contributed to data collection. V. Donoso has helped with the design of the figures. A. Bayés-Genís and J. Sanchis have revised the final version of the manuscript. J. Núñez participated in the design of the study, patient follow-up, statistical analysis, and revision of the manuscript.
CONFLICTS OF INTERESTJ. Sanchis is editor-in-chief of Rev Esp Cardiol. The journal's editorial procedure to ensure impartial handling of the manuscript has been followed. J. Núñez reports personal fees or advisory boards from Alleviant, AstraZeneca, Boehringer Ingelheim, Bayer, Novartis, NovoNordisk, Pfizer, Rovi, and Vifor Pharma (outside the submitted work). The other authors report no conflicts of interest regarding this study.