The indication for the use of cardiac implantable devices (CID) such as implantable cardioverter-defibrillators (ICD) and cardiac resynchronization therapy (CRT) was established before the approval of new treatments for heart failure (HF) with reduced ejection fraction (HFrEF).1 New treatments as sacubitril-valsartan (SV) and sodium/glucose cotransporter-2 inhibitors (SGLT2i) have been associated with left ventricular reverse remodeling (LVRR) and improved functional class,2 thus making unclear the optimal time for CID implantation since an improvement in left ventricular function or functional class with new HF treatments might be associated with the loss of CID indication.
The aim of this study was to analyze the effects of SV in modifying the indication of CID in an unselected population with chronic HFrEF and to find baseline predictors related to a change in CID indication.
Retrospective analysis of patients with symptomatic chronic HF and left ventricular ejection fraction<40% who initiated SV from September 2016 to December 2020 in our institution. Patients were treated with guideline-directed medical therapy (table 1).1 We defined patients with CID indication (ICD/CRT) before SV initiation according to current European guidelines.1 The study flowchart is shown in figure 1. We analyzed baseline characteristics, change in device indication after SV titration, and its possible clinical predictors. The reassessment of CID indication was done at least 6 months after the highest tolerated dose of SV was achieved. Following univariate analysis, predictors for CID indication lost after SV initiation were explored using logistic regression. The model was adjusted for potential confounders. Chronic kidney disease, HF etiology, time between HF diagnosis and SV initiation, baseline N-terminal probrain natriuretic peptide (NT-proBNP), left ventricular end-diastolic diameter (LVEDD), New York Heart Association functional class (NYHA) and left ventricle ejection fraction were included in the multivariate analysis. P values<.05 were considered statistically significant. Statistical analysis was performed with SPSS version 25 (IBM Corporation, USA). The study was approved by the local ethics committee (number CEIm 2019/8745).
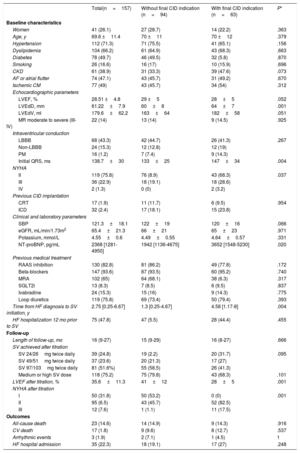
Baseline characteristics, follow-up, and outcomes of patients depending on final CID indication
| Total(n=157) | Without final CID indication (n=94) | With final CID indication (n=63) | P* | |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Women | 41 (26.1) | 27 (28.7) | 14 (22.2) | .363 |
| Age, y | 69.6 ±11.4 | 70 ±11 | 70 ±12 | .379 |
| Hypertension | 112 (71.3) | 71 (75.5) | 41 (65.1) | .156 |
| Dyslipidemia | 104 (66.2) | 61 (64.9) | 43 (68.3) | .663 |
| Diabetes | 78 (49.7) | 46 (49.5) | 32 (5.8) | .870 |
| Smoking | 26 (16.6) | 16 (17) | 10 (15.9) | .696 |
| CKD | 61 (38.9) | 31 (33.3) | 39 (47.6) | .073 |
| AF or atrial flutter | 74 (47.1) | 43 (45.7) | 31 (49.2) | .670 |
| Ischemic CM | 77 (49) | 43 (45.7) | 34 (54) | .312 |
| Echocardiographic parameters | ||||
| LVEF, % | 28.51 ±4.8 | 29 ±5 | 28±5 | .052 |
| LVEdD, mm | 61.22±7.9 | 60±8 | 64±7 | .001 |
| LVEdV, ml | 179.6±62.2 | 163±64 | 182±58 | .051 |
| MR moderate to severe (III-IV) | 22 (14) | 13 (14) | 9 (14.5) | .925 |
| Intraventricular conduction | ||||
| LBBB | 68 (43.3) | 42 (44.7) | 26 (41.3) | .267 |
| Non-LBBB | 24 (15.3) | 12 (12.8) | 12 (19) | |
| PM | 16 (1.2) | 7 (7.4) | 9 (14.3) | |
| Initial QRS, ms | 138.7±30 | 133±25 | 147±34 | .004 |
| NYHA | ||||
| II | 119 (75.8) | 76 (8.9) | 43 (68.3) | .037 |
| III | 36 (22.9) | 18 (19.1) | 18 (28.6) | |
| IV | 2 (1.3) | 0 (0) | 2 (3.2) | |
| Previous CID implantation | ||||
| CRT | 17 (1.9) | 11 (11.7) | 6 (9.5) | .954 |
| ICD | 32 (2.4) | 17 (18.1) | 15 (23.8) | |
| Clinical and laboratory parameters | ||||
| SBP | 121.3±18.1 | 122±19 | 120±16 | .066 |
| eGFR, mL/min/1.73m2 | 65.4±21.3 | 66±21 | 65±23 | .971 |
| Potassium, mmol/L | 4.55±0.6 | 4.49±0.55 | 4.64±0.57 | .331 |
| NT-proBNP, pg/mL | 2368 [1281-4950] | 1942 [1136-4675] | 3652 [1548-5230] | .020 |
| Previous medical treatment | ||||
| RAAS inhibition | 130 (82.8) | 81 (86.2) | 49 (77.8) | .172 |
| Beta-blockers | 147 (93.6) | 87 (93.5) | 60 (95.2) | .740 |
| MRA | 102 (65) | 64 (68.1) | 38 (6.3) | .317 |
| SGLT2i | 13 (8.3) | 7 (8.5) | 6 (9.5) | .837 |
| Ivabradine | 24 (15.3) | 15 (16) | 9 (14.3) | .775 |
| Loop diuretics | 119 (75.8) | 69 (73.4) | 50 (79.4) | .393 |
| Time from HF diagnosis to SV initiation, y | 2.75 [0.25-6.67] | 1.3 [0.25-4.67] | 4.58 [1.17-8] | .004 |
| HF hospitalization 12 mo prior to SV | 75 (47.8) | 47 (5.5) | 28 (44.4) | .455 |
| Follow-up | ||||
| Length of follow-up, mo | 16 (9-27) | 15 (9-29) | 16 (8-27) | .666 |
| SV achieved after titration | ||||
| SV 24/26mg twice daily | 39 (24.8) | 19 (2.2) | 20 (31.7) | .095 |
| SV 49/51mg twice daily | 37 (23.6) | 20 (21.3) | 17 (27) | |
| SV 97/103mg twice daily | 81 (51.6%) | 55 (58.5) | 26 (41.3) | |
| Medium or high SV dose | 118 (75.2) | 75 (79.8) | 43 (68.3) | .101 |
| LVEF after titration, % | 35.6±11.3 | 41±12 | 28±5 | .001 |
| NYHA after titration | ||||
| I | 50 (31.8) | 50 (53.2) | 0 (0) | .001 |
| II | 95 (6.5) | 43 (45.7) | 52 (82.5) | |
| III | 12 (7.6) | 1 (1.1) | 11 (17.5) | |
| Outcomes | ||||
| All-cause death | 23 (14.6) | 14 (14.9) | 9 (14.3) | .916 |
| CV death | 17 (1.8) | 9 (9.6) | 8 (12.7) | .537 |
| Arrhythmic events | 3 (1.9) | 2 (7.1) | 1 (4.5) | 1 |
| HF hospital admission | 35 (22.3) | 18 (19.1) | 17 (27) | .248 |
AF, atrial fibrillation; CID, cardiac implantable device; CKD, chronic kidney disease; CM, cardiomyopathy; CRT, cardiac resynchronization therapy; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; CV, cardiovascular; HF, heart failure; ICD, implantable cardioverter defibrillator; LBBB, left bundle branch block; LVEdD, left ventricle end-diastolic diameter; LVEdV, left ventricle end-diastolic volume; MRA, mineraloid receptor antagonist; MR, mitral regurgitation; NT-proBNP, N-terminal probrain natriuretic peptide; NYHA, New York Heart Association functional class; PM, pacemaker; RAAS, renin-angiotensin-aldosterone system; SBP, systolic blood pressure; SGLT2i, sodium/glucose cotransporter-2 inhibitors; SV, sacubitril-valsartan.
The data are expressed as No. (%), mean±standard deviation, or median [interquartile range].
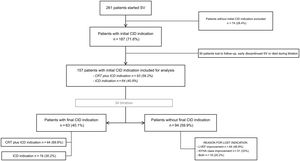
During the study period, 261 patients with symptomatic chronic HFrEF initiated SV. The baseline indication for CID was identified in 187 patients (71.6%). After excluding patients lost to follow-up, those with early discontinuation of SV and those who died during titration, 157 patients were included in the final analysis (figure 1). Patients’ baseline characteristics are described in table 1. Before SV, 93 patients (59.2%) fulfilled ESC guidelines indications1 for CRT plus ICD and 64 patients (40.8%) for ICD. After SV titration most patients lost CID indication (94; 59.9%; P<.001) (figure 1). Before SV initiation, 49 patients (31.3%) already had a CID implanted (17 CRT and 32 ICD). Of note, around 30% of those patients lost indication on follow-up. Patients who lost CID indication after SV titration had less advanced NYHA functional class, narrower QRS, smaller LVEDD and volume, and lower NT-proBNP levels when SV was initiated (table 1). Importantly, patients with shorter time between diagnosis of HF and SV initiation were more prone to lose CID indication (1.3 [0.25-4.67] vs 4.58 [1.17-8] years; P<.004). SV dose achieved after titration was not related to final CID indication (P <.101).
LVRR was the main reason for loss of CID indication (n=44; 46.8%), followed by symptoms improvement (NYHA I, n=31, 33%) or both (n=19, 20.2%). After multivariate analysis, a shorter time between HF diagnosis and SV initiation (adjusted hazard ratio (HR), 0.90; 95% confidence interval (95%CI), 0.83-0.99; P <.025), less advanced NYHA class (adjusted HR, 0.30; 95%CI, 0.13-0.70; P <.005) and smaller LVEDD (HR, 0.93; 95%CI, 0.88-0.99; P <.023) were the only independent baseline predictors for loss of CID indication. Median follow-up was 16 (9-27) months. During follow-up, there were no differences in terms of death, arrhythmic events or HF decompensations between patients who lost or maintained CID indication (table 1).
The main finding of this study is that treatment with SV significantly reduced the need of CID in patients with HFrEF. Remarkably, almost 60% of patients with an initial indication for CID lost it after treatment with SV, mainly due to LVRR, one of the major benefits of SV treatment.3,4 Recent studies with SV have demonstrated the relationship between LVRR and clinical outcomes in HFrEF patient.5 The impact on LVRR of other HF treatments is comparably lower,3,6 which may represent a minor capacity to reduce CID indication. Notably, a shorter time between HF diagnosis and SV initiation, a better NYHA class and less dilated left ventricle were independent predictors of CID indication loss. These findings highlight the importance of HF treatment optimization in the early stages of the disease to favor prompt LVRR and clinical improvement.2 Finally, the results of our study show that a strategy providing SV before consideration of a CID could potentially avoid the need for almost 60% of CIDs, decreasing the short- and long-term potential complications of cardiac devices as well as being associated with an overall lower health care expenditure without compromising patient outcomes. The main limitations of this study are its retrospective nature and the small percentage of patients treated with SGLT2i, a known drug also associated with prognostic improvement of patients with HFrEF.2 However, it is worth mentioning that most studies analyzing the potential role of SV on remodeling did not assess the role of SGLT2i,3–6 presumably because at the time they were carried out this treatment was not yet approved for HFrEF.
In conclusion, the use of SV has been associated with an almost 60% decrease in CID indication in patients with HFrEF. Because less advanced disease and shorter time to SV initiation impacts CID indication, SV should be started as soon as possible, especially before CID is considered.
FUNDINGThis research received no external funding.
AUTHORS’ CONTRIBUTIONSConceptualization, methodology, data curation, formal analysis: L. C. Belarte-Tornero and N. Farré; investigation: L. C. Belarte-Tornero, N. Farré, and D. Mojón; writing original draft preparation: L. C. Belarte-Tornero, and D. Mojón; writing review and editing: N. Farré and L. C. Belarte-Tornero; visualization: D. Mojón, E. Solé-González, S. Ruiz-Bustillo, and S. Valdivielso-More; supervision: N. Farré. All authors have read and agreed to the published version of the manuscript.
CONFLICTS OF INTERESTL.C. Belarte-Tornero reports grants, honoraria for lectures, and support for attending meetings from Novartis, Rovi, and AstraZeneca. N. Farré reports honoraria for lectures from Novartis. S. Valdivielso-More reports honoraria for educational events and support for attending meetings from Novartis.