Transthyretin cardiac amyloidosis (ATTR-CA) is a severe type of infiltrative cardiomyopathy caused by the deposition of transthyretin amyloid fibrils. The disease may be of the hereditary (ATTRv) or wild-type (ATTRwt) variant.1
Tafamidis is a transthyretin stabilizer that has been shown to increase survival and lower hospital admissions due to ATTR-CA. Diflunisal is a nonsteroidal anti-inflammatory drug that stabilizes transthyretin in vitro and has been found to delay neurologic involvement in cases of ATTRv.2 Although data on the effectiveness of diflusinal in cardiology are scarce and based on small-scale studies, the drug has been used in patients with ATTR-CA for whom no therapeutic alternatives exist.3–5 There is concern regarding chronic use of diflusinal in patients with ATTR-CA due to the potential adverse effects of this anti-inflammatory treatment in patients who tend to develop heart failure and some degree of renal failure, and who frequently take anticoagulants.
We aimed to assess the tolerability, safety, and efficacy of diflunisal as treatment for ATTR-CA.
A retrospective, longitudinal analysis was performed of patients with ATTR-CA treated with diflunisal in a Spanish hospital between June 2018 and March 2023. We evaluated treatment tolerance, electrocardiographic and echocardiographic findings, as well as the incidence of adverse events. The safety parameters studied included worsening renal function (rise in creatinine >0.3mg/dL), significant bleeding, and gastric intolerance prompting suspension of treatment.
Diflunisal was administered at a dose of 250mg every12hours to 30 patients (28 male; mean age 77.5±10 years). Of these, there were 2 with ATTRv genotypes (Val50Met and Ala65Thr) and 28 with ATTRwt. In addition, 13 (43%) had carpal tunnel syndrome, 11 (36.7%) biceps tendon rupture, and 5 (16.7%) lumbar canal stenosis. Most patients had early-stage disease (86.2% in stage I of the National Amyloid Centre staging system). At the beginning of treatment, 13 patients (43.3%) had New York Heart Association (NYHA) class I disease, 16 (53.3%) were in NYHA class II, and 1 (3.3%) in NYHA class III. Additionally, 11 patients (36.7%) had atrial fibrillation and 5 (16.7%) had pacemakers.
Twenty-two patients (73%) were treated with a proton pump inhibitor and 11 (36.6%) were undergoing anticoagulant therapy (3 with acenocoumarol and 8 with direct anticoagulants). Only 1 patient was receiving patisiran to treat polyneuropathy. Twenty patients (66.7%) were receiving diuretics, mostly low furosemide doses at a median dose of 30 [interquartile range, 10-40] mg.
The median follow-up time was 260 [interquartile range, 123-483] days. The treatment was discontinued in 7 patients (23.3%) due to adverse events and in 4 (13.3%) who enrolled in clinical trials. The treatment was withdrawn in 1 patient due to futility. There were 4 recorded bleeding episodes, of which 3 were not life-threatening (epistaxis, gingival bleeding, spontaneous hematoma of the arm) and 1 with multiple subdural hematomas resulting from several falls. Renal function significantly worsened in 8 patients (26.7%), although the treatment was only discontinued in 2 patients based on medical judgment. The drug was withdrawn in 1 patient due to paresthesia. No deaths were recorded during follow-up, and only 1 patient was admitted due to heart failure following 97 days of treatment.
An analysis of biomarker data (n=28) at a median follow-up time of 260 [interquartile range, 128-529] days revealed a slight increase in creatinine (0.92±0.16 vs 1.08±0.27mg/dL; P<.01); in N-terminal pro–B-type natriuretic peptide (NT-proBNP), with a value of 925 [281-1686] pg/mL vs 1692.5 [580.5-2438.5] pg/mL (P<.01); and in hemoglobin (14.9±1.2 vs 15.3±1.3g/dL; P=.02). We also observed a decrease in platelet count (189 [157-235]×103/μl vs 173 [136-215]×103/μl; P=.01). No change was observed in electrocardiographic (n=26; median duration of follow-up, 324 days) or echocardiographic parameters (n=14; median duration of follow-up, 453 days) (table 1).
Baseline characteristics and follow-up data for patients treated with diflunisal and those receiving no disease-specific treatment
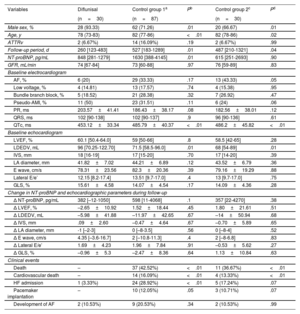
| Variables | Diflunisal | Control group 1a | Pb | Control group 2c | Pd |
|---|---|---|---|---|---|
| (n=30) | (n=87) | (n=30) | |||
| Male sex, % | 28 (93.33) | 62 (71.26) | .01 | 20 (66.67) | .01 |
| Age, y | 78 (73-83) | 82 (77-86) | <.01 | 82 (78-86) | .02 |
| ATTRv | 2 (6.67%) | 14 (16.09%) | .19 | 2 (6.67%) | .99 |
| Follow-up period, d | 260 [123-483] | 527 [183-1289] | .01 | 487 [210-1321] | .04 |
| NT-proBNP, pg/mL | 848 [281-1279] | 1630 [388-4145] | .01 | 615 [251-2693] | .90 |
| GFR, mL/min | 74 [67-84] | 73 [60-88] | .97 | 76 [59-89] | .83 |
| Baseline electrocardiogram | |||||
| AF, % | 6 (20) | 29 (33.33) | .17 | 13 (43.33) | .05 |
| Low voltage, % | 4 (14.81) | 13 (17.57) | .74 | 4 (15.38) | .95 |
| Bundle branch block, % | 5 (18.52) | 21 (28.38) | .32 | 7 (26.92) | .47 |
| Pseudo-AMI, % | 11 (50) | 23 (31.51) | .11 | 6 (24) | .06 |
| PR, ms | 203.57±41.41 | 186.43±38.17 | .08 | 182.56±38.01 | .12 |
| QRS, ms | 102 [90-138] | 102 [90-137] | .9 | 96 [90-136] | .61 |
| QTc, ms | 453.12±33.34 | 485.79±40.37 | <.01 | 486.2±45.82 | <.01 |
| Baseline echocardiogram | |||||
| LVEF, % | 60.1 [50.4-64.0] | 59 [50-66] | .8 | 58.5 [42-65] | .28 |
| LDEDV, mL | 96 [70.25-122.70] | 71.5 [58.5-96.0] | .01 | 68 [54-89] | .01 |
| IVS, mm | 18 [16-19] | 17 [15-20] | .70 | 17 [14-20] | .39 |
| LA diameter, mm | 41.82±7.02 | 44.21±6.89 | .12 | 43.52±6.79 | .36 |
| E wave, cm/s | 78.31±23.56 | 82.3±20.36 | .39 | 79.16±19.29 | .88 |
| Lateral E/e’ | 12.15 [8.2-17.4] | 13.51 [9.7-17.0] | .4 | 13 [9.7-17.0] | .75 |
| GLS, % | 15.61±4.58 | 14.07±4.54 | .17 | 14.09±4.36 | .28 |
| Change in NT-proBNP and echocardiographic parameters during follow-up | |||||
| Δ NT-proBNP, pg/mL | 382 [–12-1050] | 598 [11-4068] | .1 | 357 [22-4270] | .38 |
| Δ LVEF, % | –2.65±10.92 | 1.52±18.44 | .45 | 1.80±21.61 | .51 |
| Δ LDEDV, mL | –5.98±41.88 | –11.97±42.65 | .67 | –14±50.94 | .68 |
| Δ IVS, mm | .09±2.60 | –0.47±4.64 | .67 | –0.70±5.89 | .65 |
| Δ LA diameter, mm | -1 [–2-3] | 0 [–8-3.5] | .56 | 0 [–8-4] | .52 |
| Δ E wave, cm/s | 4.35 [–3.6-16.7] | 2 [–10.8-11.3] | .4 | 2 [–8-6.8] | .83 |
| Δ Lateral E/e’ | 1.69±4.23 | 1.96±7.84 | .91 | –0.53±5.62 | .27 |
| Δ GLS, % | –0.96±5.3 | –2.47±8.36 | .64 | 1.13±10.84 | .63 |
| Clinical events | |||||
| Death | – | 37 (42.52%) | <.01 | 11 (36.67%) | <.01 |
| Cardiovascular death | – | 14 (16.09%) | <.01 | 4 (13.33%) | <.01 |
| HF admission | 1 (3.33%) | 24 (28.92%) | <.01 | 5 (17.24%) | .07 |
| Pacemaker implantation | – | 10 (12.05%) | .05 | 3 (10.71%) | .07 |
| Development of AF | 2 (10.53%) | 9 (20.53%) | .34 | 2 (10.53%) | .99 |
AF, atrial fibrillation; ATTR-CA, transthyretin cardiac amyloidosis; ATTRv, hereditary ATTR amyloidosis; GFR, glomerular filtration rate; GLS, global longitudinal strain; HF, heart failure; IVS, interventricular septum; LA, left atrium; LDEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro–B-type natriuretic peptide; Pseudo-AMI, pseudoinfarct pattern; TAPSE, tricuspid annular plane systolic excursion.
Values indicate No. (%), mean±standard deviation, or median [interquartile range].
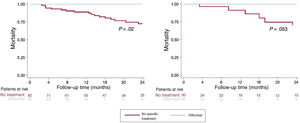
We compared the disease course in treated patients against that of all patients with ATTR-CA who did not undergo any specific treatment (and were not included in clinical trials) and who, in our facility, would have been eligible to receive diflunisal (glomerular filtration rate [GFR] ≥45mL/min) for the same time period (n=87). An additional comparison was made with a second control group matched 1:1 based on GFR (≥45mL/min), NT-proBNP (> 3000 vs ≤3000 pg/mL), and amyloidosis subtype (ATTRwt vs ATTRv) (table 1). Findings from the survival analysis showed lower mortality among patients who received diflunisal compared with the overall control group and a trend toward lower mortality compared with the matched control group (figure 1). Due to the small sample size, we were unable to match the study groups based on age.
Survival in patients with transthyretin cardiac amyloidosis according to treatment. Time to death for patients with transthyretin cardiac amyloidosis treated with diflunisal compared with a control group comprising patients having a glomerular filtration rate ≥45mL (left) and a matched control group based on glomerular filtration rate, N-terminal pro–B-type natriuretic peptide, and amyloidosis subtype (right).
In this study, we report on our experience with diflunisal as treatment for patients with ATTR-CA. Despite the smaller evidence base regarding the efficacy of the drug and its less favorable safety profile compared with that of tafamidis, treatment with diflunisal is a significantly less costly option. Use of diflunisal may be reasonable in settings where tafamidis is inaccessible for economic reasons or in patients who cannot receive the drug for other reasons, such as those with subclinical phenotypes or when there is high clinical suspicion of ATTR-CA despite no confirmed diagnosis. The largest study conducted to date of patients with ATTR-CA treated with diflunisal included 35 participants.6 The median length of follow-up was 3.2 years, and the treatment was discontinued in 14 patients (40%) due to adverse effects, most of which (57%) concerned worsening of renal function. The results of the present research are consistent with these earlier findings and confirm the need to carefully select candidates for treatment and closely monitor renal function. Data from this study reveal a lower incidence of bleeding (only 1 case), which may be attributable to the higher age of the patients in our cohort as well as the fact that 36% were receiving anticoagulant treatment. Furthermore, no significant changes in echocardiographic parameters were recorded, and our results show lower mortality among patients receiving the treatment compared with those who did not. These results should be interpreted cautiously, as nonrandomized comparative studies are inevitably subject to selection bias.
To conclude, in the cohort studied, diflunisal showed a moderate degree of tolerance, with a 23% rate of treatment discontinuation due to adverse events. The most common events were worsening of renal function, epigastric pain, and minor bleeding. Most events were mild and occurred during the first months of treatment. Despite the small sample size, no significant changes were observed in electrocardiographic or echocardiographic parameters. Rather, treatment with diflunisal was associated with a lower mortality rate compared with study patients who received no disease-specific treatment.
FUNDINGFunding for this study has been received from the Carlos III Health Institute (grants PI18/0765 & PI20/01379). This research was co-funded by the European Regional Development Fund/European Social Fund, “A way to make Europe”/“Investing in your future”. The CNIC (Centro Nacional de Investigaciones Cardiovasculares) receives funding from the Carlos III Health Institute, the Ministry of Science and Innovation, and the Fundación Pro-CNIC, and is a Severo Ochoa affiliate center (CEX2020-001041-S).
ETHICAL CONSIDERATIONSThis study was approved by the ethics committee of Hospital Puerta de Hierro, allowing retrospective use of participant data for the purposes of this research. SAGER (Sex and Gender Equity in Research) guidelines were not taken into account regarding the variables of sex and gender.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCENo artificial intelligence tools were used to conduct this research.
AUTHORS’ CONTRIBUTIONSB. Peiró-Aventín, E. Cabrera-Romero, N. Mora-Ayestarán, F. Domínguez, and E. González-López collected study data; B. Peiró-Aventín drafted the first version; E. González-López obtained funding, and P. García-Pavía supervised the study, reviewed the manuscript, and obtained funding.
CONFLICTS OF INTERESTF. Domínguez has received grants or fees from Pfizer for speaking engagements and advisory services and from Alnylam for speaking engagements. E. González-López has appeared as a speaker for events organized by Pfizer, Alnylam, and Eidos, and has received advisory fees from Pfizer, Proclara, Novo Nordisk, and Akcea. P. García-Pavía has received speaking fees from Alnylam Pharmaceuticals, AstraZeneca, Bridgebio, Intellia, Ionis Pharmaceuticals, NovoNordisk, and Pfizer; and consulting fees from Alexion, Alnylam Pharmaceuticals, AstraZeneca, ATTRalus, Bridgebio, General Electric, Intellia, Neurimmune, NovoNordisk, and Pfizer. All authors declare that their institution has received funding for research/educational activities from Alnylam Pharmaceuticals, AstraZeneca, Bridgebio, Intellia, NovoNordisk, and Pfizer. P. García-Pavía serves as Associate Editor of Revista Española de Cardiología; to ensure impartial processing of the manuscript, the appropriate editorial procedure established by the journal has been followed.