Oral anticoagulants (OAC) are the treatment of choice for preventing ischemic stroke in patients with nonvalvular atrial fibrillation (NVAF).1 However, these drugs (including the new OACs) are associated with an increased risk of serious complications such as intracranial hemorrhage (ICH).2 Restarting OACs after an ICH triples the risk of hemorrhagic events,3 therefore their use in this context is controversial and can even be contraindicated.4 In addition, there is little evidence on the safety of new OACs following ICH.4 Percutaneous closure of the left atrial appendage (LAA) is an effective therapeutic alternative to OACs.5 However, there are insufficient data on the safety and effectiveness of this procedure in patients with ICH.6
Our objective was to evaluate the safety and effectiveness of LAA closure in patients with an indication for OACs due to NVAF with a history of ICH.
The study included all patients with an indication for OACs for NVAF and a history of ICH referred to our unit between June 2009 and June 2016 for LAA closure. We analyzed clinical, echocardiographic, and procedure-related variables. The devices used for LAA closure were the Amplatzer Cardiac Plug, the Amplatzer Amulet (both St. Jude Medical), and the Watchman Implant (Boston Scientific). After the procedure, patients were treated with antiplatelet therapy or anticoagulation (low-molecular weight heparin) for at least 45 days. The decision on antiplatelet therapy vs anticoagulation was made at the discretion of the surgeon in consensus with neurology. At follow-up (at 45 days, 6 months, 12 months, and annually thereafter) we analyzed the following variables: death, ischemic stroke, and hemorrhagic events. A follow-up transesophageal echocardiogram was performed at 45 days postprocedure.
Continuous variables are presented as median [interquartile range] or mean ± standard deviation. Categorical variables are presented as frequency and percentage. A P-value < .05 was considered statistically significant.
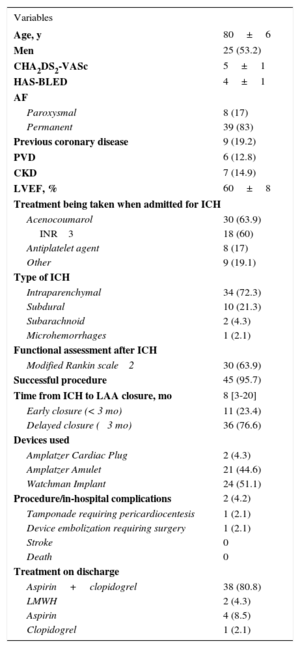
Until June 2016, 174 patients underwent percutaneous LAA closure in our hospital. The indication for closure was ICH in 47 patients (27%; 25 men and 22 women). Table 1 shows the patient and procedural characteristics. The mean age was 80 ± 6 years. The mean CHA2DS2-VASc and HAS-BLED scores were 5 ± 1 and 4 ± 1, respectively. Most patients (63.9%) were on treatment with acenocoumarol (Table 1) when the ICH occurred. Closure of LAA was successful in 95.7% of patients (45/47). Two patients developed complications during the procedure or during their hospital stay (Table 1). The most-used device was the Watchman implant (51.1%). Closure was performed at a median of 8 months [range, 3-20 months] after ICH. In 11 patients (23.4%), closure was before 3 months (early). No significant differences between the early and delayed (> 3 months) closure groups were found.
Baseline Characteristics
| Variables | |
|---|---|
| Age, y | 80±6 |
| Men | 25 (53.2) |
| CHA2DS2-VASc | 5±1 |
| HAS-BLED | 4±1 |
| AF | |
| Paroxysmal | 8 (17) |
| Permanent | 39 (83) |
| Previous coronary disease | 9 (19.2) |
| PVD | 6 (12.8) |
| CKD | 7 (14.9) |
| LVEF, % | 60±8 |
| Treatment being taken when admitted for ICH | |
| Acenocoumarol | 30 (63.9) |
| INR 3 | 18 (60) |
| Antiplatelet agent | 8 (17) |
| Other | 9 (19.1) |
| Type of ICH | |
| Intraparenchymal | 34 (72.3) |
| Subdural | 10 (21.3) |
| Subarachnoid | 2 (4.3) |
| Microhemorrhages | 1 (2.1) |
| Functional assessment after ICH | |
| Modified Rankin scale 2 | 30 (63.9) |
| Successful procedure | 45 (95.7) |
| Time from ICH to LAA closure, mo | 8 [3-20] |
| Early closure (< 3 mo) | 11 (23.4) |
| Delayed closure ( 3 mo) | 36 (76.6) |
| Devices used | |
| Amplatzer Cardiac Plug | 2 (4.3) |
| Amplatzer Amulet | 21 (44.6) |
| Watchman Implant | 24 (51.1) |
| Procedure/in-hospital complications | 2 (4.2) |
| Tamponade requiring pericardiocentesis | 1 (2.1) |
| Device embolization requiring surgery | 1 (2.1) |
| Stroke | 0 |
| Death | 0 |
| Treatment on discharge | |
| Aspirin+clopidogrel | 38 (80.8) |
| LMWH | 2 (4.3) |
| Aspirin | 4 (8.5) |
| Clopidogrel | 1 (2.1) |
AF, atrial fibrillation; CKD, chronic kidney disease; ICH, intracranial hemorrhage; INR, international normalized ratio; LAA, left atrial appendage; LMWH, low-molecular weight heparin; LVEF, left ventricular ejection fraction; PVD, peripheral vascular disease.
Values are expressed as No. (%), mean ± standard deviation, or median [interquartile range].
Median follow-up was 28 months [15-48 months]: 1 patient had an ischemic stroke with no sequelae and 1 patient had a cortical hemorrhage secondary to amyloid angiopathy. No patients died of procedure-related causes (Table 2). On the follow-up transesophageal echocardiogram, 1 patient had a device thrombus (which resolved after 1 month of treatment with low-molecular weight heparin). No patients died of device-related causes.
This study demonstrates the safety and efficiency of LAA closure in patients with an indication for OACs due to NVAF with a history of ICH. It contains the largest number of patients in a published series on this subject to date. Even with the influence of our learning curve, the procedure had a high success rate (> 95%) and a low complication rate. In addition, LAA closure was performed in the first 3 months after ICH (early) in almost 25% of patients, without complications, demonstrating its safety. At follow-up, 1 patient had an ischemic stroke (with no sequelae) at 3 years, and another patient had a cortical hemorrhage at 2 years postprocedure, secondary to amyloid angiopathy. Both were on treatment with aspirin 100 mg/d.
Fahmy et al.6 recently published a series of 24 patients with NVAF and a history of ICH who underwent LAA closure, also with a high success rate. Compared with our study, the time between ICH and LAA closure was longer (30 ± 48 months) and the follow-up time was shorter (11.9 ± 13.3 months).
Although this is an observational, single-center study with a small number of patients, it has the largest number of patients in a published series on this subject to date.
In conclusion, percutaneous LAA closure is safe and effective in patients with an indication for long-term anticoagulation for NVAF and a history of ICH, in the mid- to long-term.
FundingThis study was conducted as part of an intensified research activity program (Castile and León Regional Health Authority, Spain).
Conflicts of interestI. Cruz-González is a proctor for St. Jude Medical and Boston Scientific.