Strategies are needed to reduce health care costs and improve patient care. The objective of our study was to analyze the safety of outpatient implantation of cardioverter-defibrillators.
MethodsA retrospective study was conducted in 401 consecutive patients who received an implantable cardioverter-defibrillator between 2007 and 2012. The rate of intervention-related complications was compared between 232 patients (58%) whose implantation was performed in the outpatient setting and 169 patients (42%) whose intervention was performed in the inpatient setting.
ResultsThe mean age (standard deviation) of the patients was 62 (14) years; 336 (84%) were male. Outpatients had lower left ventricular ejection fraction and a higher percentage had an indication for primary prevention of sudden death, compared to inpatients. Only 21 outpatients (9%) required subsequent hospitalization. The rate of complications until the third month postimplantation was similar for outpatients (6.0%) and inpatients (5.3%); P = .763. In multivariate analysis, only previous anticoagulant therapy was related to the presence of complications (odds ratio = 3.2; 95% confidence interval, 1.4-7.4; P < .01), mainly due to an increased rate of pocket hematomas. Each outpatient implantation saved approximately €735.
ConclusionsOutpatient implantation of implantable cardioverter-defibrillators is safe and reduces costs. Close observation is recommended for patients receiving chronic anticoagulation therapy due to an increased risk of complications.
Keywords
The number of implantable cardioverter-defibrillator (ICD) interventions has progressively increased in recent years due to the proven efficacy in preventing sudden death and expanded indications.1–4 Because of the high cost of these devices, strategies are needed to reduce implantation-associated costs.5 Traditionally, ICDs have been implanted in an inpatient setting, mainly to monitor acute implant-related complications.6 The main objective of the present study was to evaluate the safety of outpatient ICD implantations. A secondary analysis evaluated predictive variables for the development of complications and the cost reduction achieved from performing implantations without hospitalization.
METHODSPatientsThis study involved a retrospective analysis of all ICD implantations performed in our center between October 2007 (when the day hospital for cardiology opened) and March 2012. Combined ICD/resynchronization devices were excluded from the analysis. Patients were divided into 2 groups: those with same-day implantations performed without elective hospitalization (outpatients) and those hospitalized for the procedure (inpatients). The decision to perform the implantation in an outpatient or inpatient setting was made by the physician prescribing the implant.
Implantation Protocol for the Implantable Cardioverter-defibrillatorGeneral RecommendationsFor patients on acenocoumarol therapy, discontinuation of the anticoagulant was scheduled to occur between 3 and 5 days before the intervention and low-molecular-weight heparin was begun at anticoagulant dosage (enoxaparin 1mg/kg every 12hours) as bridging therapy; the last dose of enoxaparin was administered the night before the implantation. Unless contraindicated, acenocoumarol and low-molecular-weight heparin were administered from the day after the procedure until the international normalized ratio (INR) was > 2 (at which time, heparin was discontinued). No modifications were made to the antiplatelet therapy. No patient was taking any of the new oral anticoagulants. All patients received a preoperative dose of intravenous cefazolin (or vancomycin or erythromycin in the case of allergy), with a second dose 6hours after the procedure. In our center, cephalic vein cutdown is typically used to provide access, but subclavian vein puncture is used if this approach fails. When subclavian puncture was required, a follow-up chest X-ray was taken in the first 24hours to rule out a pneumothorax. No defibrillation threshold testing was done. Device follow-up was performed 12 weeks after implantation.
Outpatient Implantation StrategyThe clinical history of the patient was reviewed, a peripheral line was obtained, and prophylactic antibiotics were administered in the day hospital before the procedure. After the intervention, patients remained under observation in the day hospital for at least 6hours until the administration of the second dose of antibiotics. Upon discharge, the patients were recommended to maintain wound compression for 24hours and to keep the wound dry for the first 48hours. In the case of a subclavian puncture approach, patients returned the following day for X-ray follow-up and for compression removal and wound evaluation. Patients were admitted if any complications were suspected.
Inpatient Implantation StrategyFollowing the implantation, patients were given bed rest and bandage compression for the first 24hours until the follow-up X-ray. Discharge was recommended at 24hours postimplantation if no complications occurred.
Analysis of ComplicationsAll complications occurring until the first device follow-up at 12 weeks were studied: device pocket hematoma (with or without the need for transfusion or hematoma evacuation), pocket or device infection, pneumothorax, hemothorax, cardiac perforation, cardiac tamponade, lead dislodgement, stroke, noncerebral embolism, or heart failure decompensation.
Cost AnalysisCost data were obtained from the Management Control Department of our center and included health personnel, intermediary and structural services, health material, and pharmaceutical products, as well as laboratory and radiology costs. All costs due to the implant itself (the cost of the device and of the electrophysiology room) were eliminated from the calculations. The Management Control Department independently analyzes costs each year and thus recommended analysis of all patients during 1 budget year to simplify the calculations and improve data homogeneity. Thus, we only analyzed the costs of the most recent year for which complete data were available at the time of our request (2010). Costs were compared between the patients with day hospital ICD implantation (including costs due to the scheduled visit on the following day and admission if required) and the patients with elective hospitalization for ICD implantation (excluding patients admitted for another cause if the ICD was implanted during this hospitalization).
Statistical AnalysisContinuous variables are presented as means (standard deviation) and were compared with the Student t test. Categorical variables are presented as proportions and were compared with the chi-square test (or Fisher exact test if any cell had a value < 5). All data showed a normal distribution (Kolmogorov-Smirnov). Propensity score matching was performed to control for a probable selection bias of patients at lower risk when choosing outpatient ICD implantation. For this approach, logistic regression was performed with outpatient implantation as the dependent variable. Independent variables consisted of those variables with P < .25 in the univariate analysis or with a plausible relationship with outpatient implantation (left ventricular ejection fraction, a primary or secondary prevention indication, first implant or generator replacement, sex, age, anticoagulant therapy, kidney failure, diabetes mellitus, chronic obstructive pulmonary disease). The resulting variable PRE_1 assigned to patients a specific probability that the implantation would be performed under hospitalization. The area under the receiver operating characteristic curve was subsequently calculated with the variable PRE_1 to verify the ability of the propensity score to predict outpatient or inpatient implantation. The value was > 0.8 (0.86), indicating excellent discriminatory ability. To evaluate the presence of independent predictors of complications, a logistic regression was performed that included all variables with P < .25 in the univariate analysis or a plausible relationship with the presence of complications, as well as the PRE_1 variable from the propensity score (and thus the model was adjusted by the probability of a patient being assigned to the outpatient or inpatient group). The Holm method was used to correct for multiple testing. A bilateral P value < .05 was considered significant. All statistical analyses were performed with SPSS software version 20.0 (SPSS Inc, Chicago, Illinois, United States).
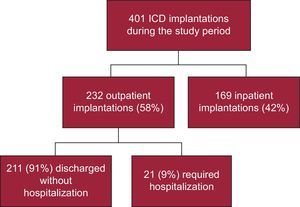
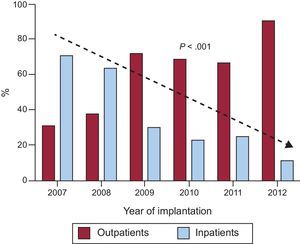
RESULTSDuring the study period, 401 ICD implantations were performed, 232 (58%) outpatient and 169 (42%) inpatient procedures (Figure 1). Of the inpatients, 91 had been admitted in a scheduled manner for the implant and 78 had been admitted for another cause (generally for ventricular tachycardia that prompted the implantation). The proportion of outpatient implantations significantly increased each year from the opening of the day hospital in 2007, with only 10% of patients hospitalized when undergoing ICD implantation in 2012 (Figure 2).
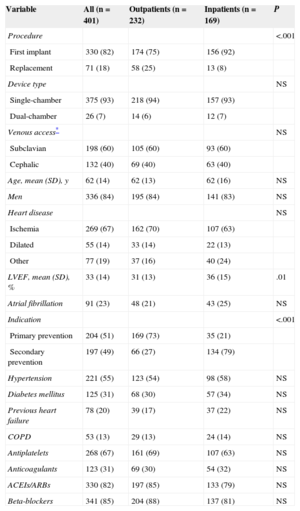
Population characteristics are shown in Table 1, as well as a comparison between outpatients and inpatients. The only significant differences between the 2 groups were that outpatients had lower left ventricular ejection fraction and a higher proportion of generator replacements and indications for primary prevention. The mean hospital stay of the inpatients was 3.9 (SD, 4.1) days, vs 1.87 [SD, 4.3] days for those whose implantation was scheduled.
Clinical and Implant Characteristics. Differences Between Outpatient and Inpatient Implantations
| Variable | All (n = 401) | Outpatients (n = 232) | Inpatients (n = 169) | P |
|---|---|---|---|---|
| Procedure | <.001 | |||
| First implant | 330 (82) | 174 (75) | 156 (92) | |
| Replacement | 71 (18) | 58 (25) | 13 (8) | |
| Device type | NS | |||
| Single-chamber | 375 (93) | 218 (94) | 157 (93) | |
| Dual-chamber | 26 (7) | 14 (6) | 12 (7) | |
| Venous access* | NS | |||
| Subclavian | 198 (60) | 105 (60) | 93 (60) | |
| Cephalic | 132 (40) | 69 (40) | 63 (40) | |
| Age, mean (SD), y | 62 (14) | 62 (13) | 62 (16) | NS |
| Men | 336 (84) | 195 (84) | 141 (83) | NS |
| Heart disease | NS | |||
| Ischemia | 269 (67) | 162 (70) | 107 (63) | |
| Dilated | 55 (14) | 33 (14) | 22 (13) | |
| Other | 77 (19) | 37 (16) | 40 (24) | |
| LVEF, mean (SD), % | 33 (14) | 31 (13) | 36 (15) | .01 |
| Atrial fibrillation | 91 (23) | 48 (21) | 43 (25) | NS |
| Indication | <.001 | |||
| Primary prevention | 204 (51) | 169 (73) | 35 (21) | |
| Secondary prevention | 197 (49) | 66 (27) | 134 (79) | |
| Hypertension | 221 (55) | 123 (54) | 98 (58) | NS |
| Diabetes mellitus | 125 (31) | 68 (30) | 57 (34) | NS |
| Previous heart failure | 78 (20) | 39 (17) | 37 (22) | NS |
| COPD | 53 (13) | 29 (13) | 24 (14) | NS |
| Antiplatelets | 268 (67) | 161 (69) | 107 (63) | NS |
| Anticoagulants | 123 (31) | 69 (30) | 54 (32) | NS |
| ACEIs/ARBs | 330 (82) | 197 (85) | 133 (79) | NS |
| Beta-blockers | 341 (85) | 204 (88) | 137 (81) | NS |
ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin II receptor blockers; COPD, chronic obstructive pulmonary disease; LVEF, left ventricular ejection fraction; SD, standard deviation.
Of the 232 outpatients, only 21 (9%) had to be hospitalized (Figure 1). The reasons for hospitalization were the following: pocket hematoma in 2 patients, pneumothorax in 1, logistic or social reasons in 3, transient ischemic attack in 1, acute pulmonary edema in 1, and decision of the treating physician (without immediate observed complication) in 13. All of these patients were discharged within 1 and 6 days after the implantation and without long-time consequences. There were no statistically significant differences between these patients and those directly discharged.
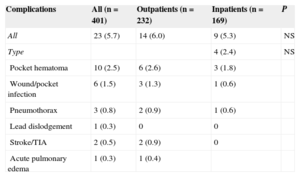
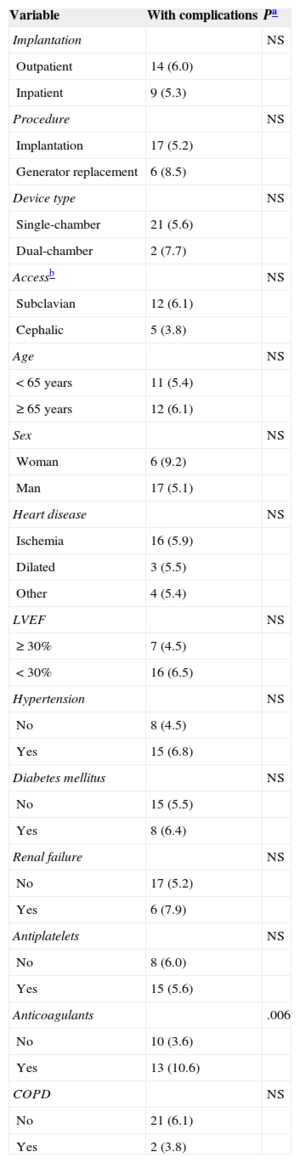
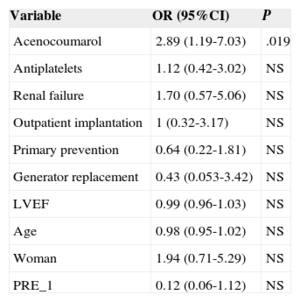
There were 23 ICD-related complications (5.7%), without significant differences between the outpatient and inpatient groups in the rate of complications (Table 2). Neither were there differences in the percentage of complications between outpatients and those inpatients whose implantation was elective (6.0% vs 5.5%; P = .853). No deaths were associated with the procedure. There were 10 wound hematomas, but only 1 patient required a blood transfusion. Two patients showed symptoms compatible with stroke (one had a history of atrial fibrillation and one had an indication for oral anticoagulation). Complete remission of symptoms occurred in less than 24hours in both patients. The only patient requiring lead relocation was in the inpatient group. Analysis of the relationship between variables (as indicated in “Methods”) and the presence of complications is shown in Tables 3 and 4 (univariate) and (multivariate). The only factor related to complications was treatment with oral anticoagulants.
Overall Complications and Comparisons Between the Patient Groups
| Complications | All (n = 401) | Outpatients (n = 232) | Inpatients (n = 169) | P |
|---|---|---|---|---|
| All | 23 (5.7) | 14 (6.0) | 9 (5.3) | NS |
| Type | 4 (2.4) | NS | ||
| Pocket hematoma | 10 (2.5) | 6 (2.6) | 3 (1.8) | |
| Wound/pocket infection | 6 (1.5) | 3 (1.3) | 1 (0.6) | |
| Pneumothorax | 3 (0.8) | 2 (0.9) | 1 (0.6) | |
| Lead dislodgement | 1 (0.3) | 0 | 0 | |
| Stroke/TIA | 2 (0.5) | 2 (0.9) | 0 | |
| Acute pulmonary edema | 1 (0.3) | 1 (0.4) |
NS, not significant; TIA, transient ischemic attack.
Values express n (%).
Univariate Analysis of Predictors of Complications by Implantation Type
| Variable | With complications | Pa |
|---|---|---|
| Implantation | NS | |
| Outpatient | 14 (6.0) | |
| Inpatient | 9 (5.3) | |
| Procedure | NS | |
| Implantation | 17 (5.2) | |
| Generator replacement | 6 (8.5) | |
| Device type | NS | |
| Single-chamber | 21 (5.6) | |
| Dual-chamber | 2 (7.7) | |
| Accessb | NS | |
| Subclavian | 12 (6.1) | |
| Cephalic | 5 (3.8) | |
| Age | NS | |
| < 65 years | 11 (5.4) | |
| ≥ 65 years | 12 (6.1) | |
| Sex | NS | |
| Woman | 6 (9.2) | |
| Man | 17 (5.1) | |
| Heart disease | NS | |
| Ischemia | 16 (5.9) | |
| Dilated | 3 (5.5) | |
| Other | 4 (5.4) | |
| LVEF | NS | |
| ≥ 30% | 7 (4.5) | |
| < 30% | 16 (6.5) | |
| Hypertension | NS | |
| No | 8 (4.5) | |
| Yes | 15 (6.8) | |
| Diabetes mellitus | NS | |
| No | 15 (5.5) | |
| Yes | 8 (6.4) | |
| Renal failure | NS | |
| No | 17 (5.2) | |
| Yes | 6 (7.9) | |
| Antiplatelets | NS | |
| No | 8 (6.0) | |
| Yes | 15 (5.6) | |
| Anticoagulants | .006 | |
| No | 10 (3.6) | |
| Yes | 13 (10.6) | |
| COPD | NS | |
| No | 21 (6.1) | |
| Yes | 2 (3.8) |
COPD, chronic obstructive pulmonary disease; LVEF, left ventricular ejection fraction; NS, not significant.
Values express n (%).
Multivariate Analysis (Logistic Regression) of Predictors of Complications
| Variable | OR (95%CI) | P |
|---|---|---|
| Acenocoumarol | 2.89 (1.19-7.03) | .019 |
| Antiplatelets | 1.12 (0.42-3.02) | NS |
| Renal failure | 1.70 (0.57-5.06) | NS |
| Outpatient implantation | 1 (0.32-3.17) | NS |
| Primary prevention | 0.64 (0.22-1.81) | NS |
| Generator replacement | 0.43 (0.053-3.42) | NS |
| LVEF | 0.99 (0.96-1.03) | NS |
| Age | 0.98 (0.95-1.02) | NS |
| Woman | 1.94 (0.71-5.29) | NS |
| PRE_1 | 0.12 (0.06-1.12) | NS |
95%CI, 95% confidence interval; LVEF, left ventricular ejection fraction; NS, not significant; OR, odds ratio; PRE_1, the variable obtained in the propensity score, which assigns to patients a specific probability of being hospitalized for the implantation.
In the cost analysis, the mean cost in 2010 (excluding electrophysiology room costs and the price of the device) was €122 (SD, €148) for each implantation performed in the day hospital and €857 (SD, €412) for elective implantations with hospitalization (SD, €452 [€289] per day of hospitalization). Thus, each implantation performed in an outpatient setting saved approximately €735.
DISCUSSIONThe usual approach for ICD implantation is to admit the patient in order to identify possible complications.6 Given the growing number of implants placed in recent years and because the cost-benefit of the devices has been disputed in some studies,7,8 cost-reducing strategies are necessary. In our experience, outpatient implantation of ICDs is safe. Because this safety also has been noted by physicians prescribing the implant, the number of outpatient procedures has progressively increased to represent approximately 90% of all implantations (Figure 2).
The characteristics of the study population are similar to those of other registries.1 Most implants were prescribed for primary prevention, and ischemia was the most common underlying heart disease. Secondary prevention was more common in the inpatient setting because the implantation often occurred after hospitalization for an arrhythmic episode. On the other hand, the left ventricular ejection fraction was lower in the outpatient group because most implantations in this group were for primary prevention, which generally requires a lower left ventricular ejection fraction.
The published incidence of complications after ICD implantation varies according to the type of complication included in the analysis. In our series, the incidence (5.7%) was similar to that described in other patient series.9,10 Various studies have reported the safety of the outpatient implantation of pacemakers.11–13 The evidence is scarcer for ICDs. In a prospective study of 71 patients randomized to same-day discharge or hospitalization for 24hours, there were no differences between the groups in the incidence of complications.14 However, that study comprised a selected low-risk population (with exclusion of subclavian puncture implantations, pacemaker dependency, and oral anticoagulant indications). Our series comprised a less selected population but there were still no differences in the incidence of complications between the outpatient and inpatient groups, despite including higher-risk patients.
In another recent study with a design and number of patients similar to ours (although only concerning implantations for primary prevention), Darda et al15 assessed the safety of outpatient implantations in a group of 198 patients discharged the same day, reporting an incidence of complications of 3% (without observing differences from a group of similar patients hospitalized for implantation). The authors commented that implantation-related complications either manifest in the short observation period afterward or appear much later, indicating that hospitalization fails to provide any relevant benefit. The incidence of pocket hematoma in that study was almost identical to that of our study (2.8% vs 2.6%, respectively). The incidence of device infection of 1.3% in our group of outpatients is also similar to that described elsewhere16,17 and was lower than that of the inpatients (1.8%). The incidence of infection may be reduced because the patients avoid the hospital environment and exposure to nosocomial gems following the implantation.
Due to earlier mobilization in those discharged on the day of the implantation, another possible complication is lead dislodgement, but in our series and 2 other studies,14,15 there was no increase in the need for lead relocation. Indeed, Choudhuri et al14 found no differences between outpatients and inpatients when using remote monitoring of the lead parameters. A rate of complications of only 1.8% was shown in the results of a recently published registry of data from the National Cardiovascular Data Registry (NCDR) containing more than 200 000 ICD implants in the United States.18 However, the authors themselves recognized that these results underestimated the true rate of complications, precluding a comparison with our results. First, unlike in our study, they only recorded inhospital complications, omitting those occurring after discharge (eg, notably, they did not include a single case of endocarditis). Moreover, the data collection method used limited the collection of information on complications, compared with the complete patient medical history used in our study. Thus, as already mentioned, their rate of hematomas of 0.3% is much lower than that seen in most series (surely indicating that the registry only recorded the most serious complications).
The only predictor of complications was treatment with oral anticoagulants, as in the series of Darda et al.15 In the present study, anticoagulant therapy was terminated and heparin was used as bridging therapy. This management strategy for patients with chronic anticoagulation has been related to increased complications from inpatient pacemaker implantations, compared with maintaining oral anticoagulation.19–21 Maintenance of oral anticoagulants could reduce adverse events after outpatient ICD implantation, but this hypothesis remains to be studied. Another alternative for possible study is whether use of the new oral anticoagulants modifies the presence of complications following cardiac device implantation. Regardless, patients with oral anticoagulant indication appear to be an at-risk population and should thus be handled with care.
Finally, given the growing number of implants and the current economic situation, there is a need for strategies that optimize health care resources. In our center, each implantation cost approximately €735 euros more with elective hospitalization in 2010. Extrapolation of that cost difference to the 232 outpatient implantations in our center that year represents a total saving of €170 520. Thus, outpatient ICD implantation appears to be a safe and promising strategy for reducing the costs associated with postimplantation hospitalization. The technique is also constantly being made simpler and cheaper: the first epicardial ICD implants were performed under surgery, but most implants are currently performed in electrophysiology rooms1 and without the need for defibrillation threshold testing.22 Further improvements have been obtained from strategies already in use, such as remote monitoring23,24 and subcutaneous defibrillators.25,26
LimitationsThe main limitation of this study is the lack of randomization: patients who received an ICD in the day hospital could have had less risk and, therefore, fewer complications. A propensity score was used to control for this selection bias, as described in “Methods”. In the present analysis, all implantation types were included (replacements, first implants, lead relocations), both outpatient and inpatient, to obtain the highest number of patients possible and capture the widest clinical spectrum. The proportion of dual-chamber ICD implants was low (only 7%), limiting the application of the results to these types of implants. Another limitation is that the study was performed in a single center with ample experience in the implantation of these devices, impeding the extrapolation of these results to centers with other characteristics. Moreover, use of the cephalic vein approach may have reduced the rate of complications in our series of patients, although only the subclavian approach was used in the recently published study mentioned above and their data are similar.15 Nonetheless, the cephalic approach could only be used in 40% of patients, possibly due to the thickness of the ICD lead, which is greater than that of pacemakers. In addition, because the benefit of the evaluation of the defibrillator threshold of the ICD following implantation remains unclear,22 no such testing is systematically performed in our center, which could affect the extrapolation of our results to patients who undergo this threshold testing.
CONCLUSIONSOutpatient ICD implantation reduces implant-related costs without increasing the rate of complications. The only predictor of complications is oral anticoagulant therapy, indicating a need for optimized management of these patients.
CONFLICTS OF INTERESTSNone declared.
We wish to thank Alfonso Cuadrado Rodríguez of the Management Control Department for his help in analyzing the economic costs.