At the start of the 20th century, cardiovascular disease (CVD) caused about 10% of all worldwide deaths; a century later, this figure had increased to 30%, with up to 80% of these events occurring in low- and middle-income countries.1
In 2006, Valentín Fuster was named president of the World Heart Federation and became a first-hand witness to the devastating impact and socioeconomic consequences of CVD, particularly in low-income countries. These experiences prompted him to write the article entitled, “Low priority of cardiovascular and chronic diseases on the global health agenda: a cause for concern”, in which he discussed the epidemiological impact of CVD and made a series of recommendations for combating its growing worldwide incidence.2 In the article, he urged the United Nations to change the name of the Millennium Development Goal “Combat HIV/AIDS, malaria, and other diseases” to “Combat infectious diseases such as HIV/AIDS and malaria, along with chronic diseases such as cardiovascular disease, diabetes mellitus, and cancer, using an integrated approach” and called for increased investment in the prevention of nontransmissible diseases: “Much can be done with limited resources, but nothing can be done with nothing”. That moment signified the formal initiation of a polypill project for secondary prevention.
The cardiovascular pandemic of the 21st century exhibits notable parallels with the global landscape of HIV in the 1990s. The antiretroviral polypill was proposed at the turn of the millennium as a public health care strategy for containing a transmissible disease affecting patients around the world, particularly those in low- and middle-income countries with limited access to an effective antiretroviral therapy. Two decades later, the antiretroviral polypill has effectively controlled the HIV pandemic based on 3 simple features: accessibility, adherence, and cost-effectiveness.
The current panorama for CVD mortality requires a similar strategy to contain what has become the leading cause of death worldwide, with more than 17 million deaths in 2011 and expectations of 24 million deaths in 2030.3 The health care, social, and economic impact of this situation demands simple solutions to curb the severe burden. The cardiovascular polypill represents one of the available tactics and must be integrated within a global cardiovascular prevention strategy.
THE POLYPILL PARADOX: A POPULATION HEALTH STRATEGY IN THE ERA OF PERSONALIZED MEDICINEIn low-income countries, the increase in cardiovascular risk factors has had a major impact on the incidence of CVD, which is compounded by the limited availability of effective treatments (both in terms of the management and treatment of acute events and of the use of drugs for the long-term prevention of CVD). Awareness of the distressing lack of access to treatment all over the world was raised by the results of the Prospective Urban Rural Epidemiological (PURE) study, which assessed rates of previous CVD (coronary heart disease or stroke) and the use of secondary prevention medication of proven efficacy and medication to reduce blood pressure in individuals aged between 35 and 70 years in rural and urban communities in countries at various stages of economic development.4 The results showed that 80.2% of patients were not receiving any cardioprotective treatment. Overall, few patients with CVD were taking antiplatelet agents (25.3%), beta-blockers (17.4%), angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin II receptor blockers (ARBs) (19.5%), or statins (14.6%). Even in high-income countries, although the use of these drugs was higher, the overall use was suboptimal (antiplatelet agents, 62.0%; beta-blockers, 40.0%; ACEIs or ARBs, 49.8%; and statins, 66.5%), while their use was even lower in low-income countries (8.8%, 9.7%, 5.2%, and 3.3%, respectively).4
The situation is different in middle-income countries: as systems for the acute treatment of events become more effective, they reduce cardiovascular mortality. This, in turn, considerably increases the prevalence of secondary prevention patients requiring effective treatments to minimize complications and recurrent events.5 However, even though most patients are discharged with an appropriate prescription for secondary prevention medication,6 their adherence falls drastically, with only 45% still taking preventive medication at 1 year.7 It is in this context that the polypill is proposed as a first step or foundational treatment for the subsequent more personalized management of the residual risk of secondary prevention patients.8
SCIENTIFIC DEVELOPMENTFrom the outset, the scientific development of the polypill had to address major questions: indication, composition, and scientific development plan.9
The indication for the polypill developed in Spain is clear: a substitute for secondary prevention patients. This decision, which may appear minor, has major implications, such as how to identify the profile of patients who could receive the polypill (in primary prevention, there are different risk thresholds) and the treatments that must be received by these patients: all active ingredients contained in the CNIC polypill (aspirin, ramipril, and atorvastatin) have a class I A indication in all treatment guidelines for secondary prevention patients.10
The work to provide data supporting the efficacy of the polypill in secondary prevention began in 2007 with the aim of studying the prevalence of adherence, understanding the causes of poor treatment adherence specifically in secondary prevention, and demonstrating the advantages of the polypill as a strategy to boost treatment adherence. Subsequently, in 2013, a major insurer in the United States supported a study directed by Dr. Fuster that was conducted to identify the correlation of treatment adherence with the risk of a major cardiovascular event in patients after an acute myocardial infarction (AMI). The evidence from these 2 studies, together with other results obtained by other groups, provided the scientific basis for the proposed SECURE trial, which was presented at a 2014 meeting of H2020; the study received funding and its results were presented at a Late Breaking Trials session of the European Society of Cardiology congress in 2022 and published on the same date in the New England Journal of Medicine (figure 1).11
FOCUS study: the polypill improves treatment adherenceThe FOCUS trial was conducted in 5 countries (Spain, Italy, Argentina, Brazil, and Paraguay) to shed light on the factors impeding proper adherence to cardiovascular therapy in a cohort of post-AMI patients, as well as to investigate the effects of the polypill on adherence and risk factor control.7
Phase 1 of the FOCUS trial studied 2118 patients to elucidate the factors interfering with proper adherence to cardiovascular treatment for secondary prevention after an AMI. In addition, 695 patients from Phase 1 were randomized to a clinical trial (FOCUS Phase 2) to compare a polypill (containing aspirin 100mg, simvastatin 40mg, and ramipril 2.5, 5, or 10mg) with the 3 drugs given separately in terms of adherence, blood pressure, and low-density lipoprotein-cholesterol (LDL-C), in addition to safety and tolerability, over 9 months. The primary endpoint was defined as treatment adherence at the final visit measured using the Morisky-Green questionnaire, as well as a pill count.
In Phase 1, adherence to the cardiovascular medication by the Morisky-Green method was 45.5%. In a multivariable regression analysis, the risk of risk of being nonadherent was associated with age younger than 50 years, depression, a complex medication regimen, low levels of social support, and worse health insurance coverage, with consistent results among all countries. In Phase 2, the polypill group exhibited a significant increase in adherence levels after 9 months compared with the group assigned to receive the 3 drugs separately: 50.8% vs 41% (P=.019; intention-to-treat population) and 65.7% vs 55.7% (P=.012; per-protocol population) for the primary endpoint of attending the final visit and with adherence measured using the combination of the Morisky-Green score and pill count. Adherence levels were also significantly higher in the polypill group when only the Morisky-Green method was used (68% vs 59%; P=.049).7
The study found no significant differences in secondary endpoints defined as systolic blood pressure (129.6 vs 128.6mmHg), mean LDL-C values (89.9 vs 91.7mg/dL), serious adverse effects (23 [6.6%] vs 21 [6%]), and death (1 case, 0.2% in each group).
In sum, in secondary prevention after an AMI and compared with 3 drugs given separately, the polypill strategy significantly increases treatment adherence measured using both methods, which indicates the usefulness of this strategy.
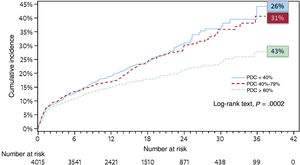
Impact of treatment adherence on CVDA study supported by a large American insurer and authored by Bansilal et al.12 investigated the relationship between medication adherence and long-term major adverse cardiovascular events (MACE) (composite of all-cause death, myocardial infarction, stroke, or coronary revascularization) in patients following an AMI and those with atherosclerotic disease. By using electronic pharmacy prescriptions to calculate the proportion of days covered with statins and ACEIs, patients were stratified by the proportion of days covered as fully adherent (80%), partially adherent (40%-79%), or nonadherent (<40%). The study compared the incidence of the primary endpoint and hospitalization rates among groups. In the post-AMI cohort, which included 4015 adults who initiated both statins and ACEIs, just 43% of the patients were classified as fully adherent, while 31% were deemed partially adherent and 26% nonadherent. In addition, the results showed that secondary prevention patients must maintain a very high level of adherence (higher than 80%) to prevent a secondary cardiovascular event. The fully adherent patients had a significantly lower risk of MACE than the partially adherent patients (a risk reduction of 19%) and the nonadherent patients (a risk reduction of 27%) (figure 2). No significant differences were found in the observed risk between the nonadherent and partially adherent groups. Moreover, compared with partial and nonadherence, full adherence was associated with a reduction in per-patient annual direct medical costs associated with hospitalizations for AMI of $369 and $440 and for revascularization procedures of $539 and $844, respectively.
Impact of treatment adherence on major clinical events in secondary prevention. Adapted with permission from Bansilal et al.11 PDC, proportion of days covered.
Data from European cohorts using absolute and relative risk assessments show that a considerable proportion of all CVD events (9% in Europe) could be attributed to poor adherence to cardioprotective medication.13 The topic is especially pertinent in wealthier nations, which provide greater access to health care systems and have higher utilization rates, and a further increase in medication effectiveness would largely require improved adherence. These data raise major questions about which practical measures should be taken by cardiologists, general physicians, nursing staff, and other people who attend to these patients to guarantee their adherence to the prescribed regimens. Adherence is boosted by patient participation in the medication prescription decision, counseling, and the strict patient monitoring to control adverse effects, but these approaches can be complicated and costly and are just one part of the solution. Predictive models indicate that interventions that reduce patients’ pill burden, particularly the cardiovascular polypill for secondary prevention, when used in conjunction with other approaches, are promising ways to improve adherence and, ultimately, patients’ health care outcomes.
NEPTUNO study: analysis of the real-life effectiveness of the polypillThe observational, retrospective, and real-world NEPTUNO trial used electronic health record data from various autonomous communities in Spain to compare the effect of the polypill on the incidence of recurrent MACE and risk factor control in 6456 patients with established atherosclerotic CVD to 3 other cohorts: a) the same monocomponents as the polypill but taken separately; b) equipotent components; and c) other drug therapies not included in the previous cohorts. After a 2-year follow-up, the risk of recurrent MACE was lower in the CNIC polypill cohort than in the control groups (22% vs the monocomponent group [P=.017]; 25% vs the equipotent group [P=.002]; and 27% vs the other therapies group [P=.001])14 (figure 3). The incremental proportion of patients achieving strict blood pressure control <130/80mmHg was higher in the CNIC polypill cohort than in all other groups (+12.5% vs +6.3% in the monocomponent group [P<.05]; +12.5% vs +2.2% in the equipotent group [P<.01]; and +12.5% vs +2.4% in the other therapies group [P<.01]). Similarly, the incremental proportion of patients achieving the LDL-C target <70mg/dL was higher in the CNIC polypill group (+10.3% vs +4.9% in the monocomponent group [P<.001]; +10.3% vs +5.7% in the equipotent group [P<.001]; and +10.3% vs +4.9% in the other therapies group [P<.001]). Finally, medication persistence at the end of the study was significantly higher in the polypill-treated patients (72.1% vs 62.2%, 60.0%, and 54.2%, respectively; P<.001). This real-world observational study was the first to demonstrate that the use of the CNIC polypill was associated with a significant reduction in the incidence of recurrent MACE, together with a delay in time to event, in a large sample of patients with a history of established atherosclerotic CVD compared with 3 different active treatment groups. These findings underscore the effectiveness of the polypill strategy for secondary cardiovascular prevention in clinical practice.
Effectiveness of the cardiovascular polypill in Spain. Results of the real-world NEPTUNO study.14
95%CI, 95% confidence interval; CV, cardiovascular; HR, hazard ratio; P, statistical significance.
The results of NEPTUNO were published about 1 year before the final visit of the last patient of the SECURE study and augured good results, given that the objectives were similar and that the effectiveness of a polypill can probably be better measured in a real-world study setting than in a prospective clinical trial (because the adherence of the control patients is not altered by their mere participation in a trial).
Effectiveness of the polypill in secondary prevention: the SECURE studyStrictly speaking, the SECURE trial was not a prerequisite for formalizing the approval of the polypill by regulatory agencies, given that the approval process simply required demonstration of a certain range of bioequivalence of the polypill components in pharmacokinetic and pharmacodynamic studies. However, the concept of the polypill has, since its early days, been subject to some resistance by the scientific community for reasons already explained; thus, a randomized prospective clinical trial was probably needed to provide definitive evidence indicating the usefulness of the polypill in secondary prevention and thereby support its implementation.
The clinical protocol of the SECURE trial was submitted in 2014 to a H2020 call entitled, “Comparing the effectiveness of existing health care interventions in the elderly”. This H2020 program outlined the framework for presenting the funding project by explaining the intricacies of providing effective health care to the elderly population. This population is susceptible to multiple comorbidities and associated polypharmacy, as well as problems related to health care access and adherence. In addition, whereas older patients are overrepresented in terms of patient number, this group is underrepresented or even excluded from many clinical trials generating the evidence base for health care interventions. Given this context, the polypill probably represents one of the best strategies for responding to this call. The European Union, through the H2020 program, provided funding of about €6 million for the SECURE trial in January 2015.
SECURE included 2499 secondary prevention patients from 113 hospitals in 7 European countries (Spain, Italy, France, Germany, Hungary, Poland, and the Czech Republic). The patients had to have had a type I AMI and be older than 75 years or be older than 65 and also have an additional risk factor (diabetes, renal failure, a previous atherosclerotic event). Notably, the mean age of the population was 75 years, about 40% had diabetes, 35% had chronic kidney disease, and more than a third had had at least 1 atherosclerotic event before the AMI. Moreover, the median time from the event to inclusion in the SECURE trial was just 7 days, suggesting that the polypill treatment usually began before hospital discharge. The trial attempted as best possible to reproduce the real-world clinical setting, so that the patients randomized to the polypill treatment (which allowed treatment with 6 formulations: aspirin 100mg, ramipril 2.5/5/10mg, and atorvastatin 20/40mg) could additionally receive antihypertensive therapy or another lipid-lowering agent other than statins, depending on their medical needs. In the control group, the treatment was discretionary according to clinical practice guidelines and, indeed, more than 40% of the patients in this group received higher potency statins than the atorvastatin 40mg received by most polypill recipients.
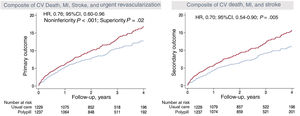
The results demonstrated an undeniable benefit in favor of the polypill in the composite primary endpoint of cardiovascular death, infarction, stroke, or urgent revascularization with a relative risk reduction of 24%, as well as a relative risk reduction for cardiovascular death of 33% (figure 4). In addition, a benefit was found in all prespecified groups of patients by country of origin, age, sex, presence or absence of diabetes, presence or absence of renal failure, and the occurrence or nonoccurrence of atherosclerotic events before study entry. This consistent benefit in the subgroups strengthened even further the primary and secondary endpoint results.
Central illustration. Primary endpoint results for the SECURE trial. Effectiveness of the polypill in the relative risk reduction for the occurrence of the primary (A) and secondary (B) endpoints.
95%CI, 95% confidence interval; CV, cardiovascular; HR, hazard ratio; MI, myocardial infarction.
Low treatment adherence has major health care and economic effects and is associated with a failure to achieve therapeutic targets and with higher rates of hospitalization and death.15
Part of the economic burden of CVD springs from a lack of therapeutic effectiveness due to poor adherence. Indeed, in the United States, the direct and indirect costs linked to poor adherence were calculated to be about $528.4 billion in 2016, with a plausible range from $495.3 to $672.7 billion, which is equivalent to 16% of the total health care spend of the United States in 2016.16 In Europe, this figure has been estimated to be €125 billion in terms of hospitalizations, emergency department care, and avoidable outpatient visits.
In Spain, a report commissioned by FarmaIndustria in 2016 17 revealed the results of a model evaluating the impact of adherence in Spain on CVD based on a review of the literature of economic assessments and studies relating adherence to effectiveness.
The results, with a time horizon of 15 years, are striking and can be summarized in 3 key points:
- 1.
Mortality and cardiovascular event rates are higher in nonadherent patients. Per-patient cost is also higher.
- 2.
Modification of the adherence levels of the nonadherent population reveals the impact of this approach on health care outcomes and the associated costs. Notably, the model demonstrates that an increase of just 1 percentage point in the mean adherence level of the nonadherent patients would represent a direct avoidable health care expenditure of almost €11 million, in addition to more than 1200 deaths and 1100 cardiovascular events avoided.
- 3.
Similarly, when the same analysis is performed but with a level of adherence increase of 10 percentage points, the direct avoidable health expenditure reaches €75 million and cardiovascular deaths and events would be reduced by 8778 and 7650, respectively.18
Poor treatment adherence represents a major economic burden for health care systems. The current research evaluating the economic impact of treatment adherence is slight and of variable quality and does not provide parameterized data that can impact public health policies. The correlation between the increased lack of adherence and higher disease prevalence must be communicated to the relevant politicians to help to reduce avoidable costs for the health care system.
Research shows that polypill-based regimens for the primary and secondary prevention of CVD are cost-effective in all regions of the world, except Sub-Saharan Africa.19 In addition, we can avail ourselves of the results of 2 studies that used data from the United Kingdom and Spain to analyze the cost-effectiveness of the polypill strategy in secondary prevention.20,21 A Markov-based cost-effectiveness analysis informed by systematic reviews (which identified effectiveness, usefulness, and adherence data) was used to assess the economic and health care system benefits of adherence to a polypill (containing aspirin 100mg, atorvastatin 20mg, and ramipril 2.5, 5, or 10mg) in the secondary prevention of cardiovascular events.
These studies estimated that a 10 percentage point uptake of the polypill in the United Kingdom would prevent 3260 cardiovascular events and 590 cardiovascular deaths over a decade.20 In Spain, the model showed that, during a 10-year period, the use of the cardiovascular polypill instead of individual components would simultaneously avoid 46 nonfatal cardiovascular events and 11 fatal events for every 1000 patients treated. The polypill would also be a more effective and cheaper strategy. Probabilistic analysis of the base case found a 90.9% probability that the polypill would be a cost-effective strategy compared with multiple monotherapies at a willingness-to-pay of €30 000 per quality-adjusted life year.21
Accordingly, the data indicate that the polypill, as well as being a clinically effective strategy, is an economical way to avoid fatal and nonfatal cardiovascular events.
Various studies have investigated patients’ preferences and acceptability of the polypill, with highly consistent results in favor of its use vs separate medications.22,23 These studies concluded that secondary prevention patients treated with a cardiovascular polypill show greater satisfaction and significantly better medication adherence than patients treated with the individual monocomponents. The patients treated with the polypill consider it a convenient treatment that is associated with a high degree of confidence and prefer it to the separate monocomponents. In addition, most patients treated with individual monocomponents express their preference for a polypill treatment when they are explained the concept.
When patients are asked for their preferences, they weigh up the treatment options: this increases their degree of involvement and commitment and their satisfaction level, which enhances treatment duration and adherence and thereby potentially leads to better treatment outcomes.
FUTURE DIRECTIONSThe polypill represents a major step toward the treatment optimization of secondary prevention patients as an effective delivery system for overall cardioprotective treatments, whether due to its ability to cost-effectively deliver medication or as a tool for optimizing treatment adherence. However, in contrast to conditions such as HIV, asthma, and migraine, for which combined treatments have earned universal acceptance, the idea of a polypill for the prevention of CVD is not always well accepted by specialist physicians, despite being popular with patients. The evidence presented in the SECURE trial should be translated into better implementation of the polypill in clinical practice in countries with polypill availability and promote the adoption of this strategy by countries in which it is not yet available. The World Heart Federation is currently immersed in the drafting of a “Roadmap for the implementation of the polypill” as a strategic priority to guarantee patients in secondary prevention access to effective treatment. This roadmap will be published in June 2024.
CONCLUSIONSThe world is facing an epidemic of nontransmissible diseases, with more than 80% of premature deaths due to CVD occurring in low- and middle-income countries. More than 30 million people around the world do not have access to appropriate secondary prevention, mainly those in countries with fewer resources. Accordingly, the availability and use of an affordable polypill would be key to reducing the numbers of deaths caused by nontransmissible diseases. The implementation of a polypill in secondary prevention as a central strategy to guarantee the worldwide optimization of cardioprotective treatment has become a requirement that should be prioritized to ensure proper access around the world, given the recent evidence supporting its clinical benefit.
FUNDINGThe present work has not received funding.
AUTHORS’ CONTRIBUTIONSBoth authors contributed equally to the drafting of this manuscript.
CONFLICTS OF INTERESTJ.M. Castellano has received honoraria from Ferrer, Pfizer, Daiichi Sankyo, and Servier. V. Fuster has no conflicts of interest to declare.