It has been demonstrated that percutaneous closure of the left atrial appendage (LAA) is an alternative to oral anticoagulation (OAC) with coumarins in patients with nonvalvular atrial fibrillation (AF), especially in those with a contraindication. However, the latest European guidelines on AF1 have not changed the previous grade of recommendation for LAA occlusion and they have retained a class IIb indication and level of evidence B for patients with a long-term contraindication for OAC due to untreatable bleeding problems. The justification for this decision lies in the high real-world complication rates, which are based on the analysis of insurance company databases, systematic reviews, and the lack of current data on LAA occluders compared with the new direct OACs for embolic prevention (sections 9.3.1 and 15.6 of the guidelines). In addition, the guidelines recognize other gaps in the evidence, such as the role of LAA occlusion in managing patients who have already experienced bleeding or stroke (section 15.7) or after intracranial hemorrhage (section 9.4.3).
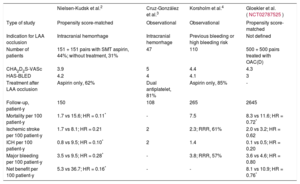
Several articles2–4 have been recently published that address these aspects and offer guidance on clinical decision making. Table 1 shows their main characteristics and results. Although these studies are observational single cohort studies or propensity score-matched control group studies, they provide valuable information in fields as complex as embolic prevention after bleeding (especially after intracranial hemorrhage) or very high risk of bleeding. In general, they demonstrate the efficacy and safety of LAA occlusion compared with the standard treatment of these patients (many of whom are without OAC due to their bleeding risk). Even the 2 matched control group studies (Nielsen-Kudsk et al.2 and Gloeker et al. [NCT02787525]) demonstrate reductions in overall mortality. Another common finding is the wide variability in pharmacological treatment after LAA occlusion, reflecting the heterogeneity of patients with bleeding or at high risk of bleeding.
Recently Published Studies on Percutaneous Closure of the Left Atrial Appendage
| Nielsen-Kudsk et al.2 | Cruz-González et al.3 | Korsholm et al.4 | Gloekler et al. (NCT02787525) | |
|---|---|---|---|---|
| Type of study | Propensity score-matched | Observational | Observational | Propensity score-matched |
| Indication for LAA occlusion | Intracranial hemorrhage | Intracranial hemorrhage | Previous bleeding or high bleeding risk | Not defined |
| Number of patients | 151 + 151 pairs with SMT aspirin, 44%; without treatment, 31% | 47 | 110 | 500 + 500 pairs treated with OAC(D) |
| CHA2D2S-VASc | 3.9 | 5 | 4.4 | 4.3 |
| HAS-BLED | 4.2 | 4 | 4.1 | 3 |
| Treatment after LAA occlusion | Aspirin only, 62% | Dual antiplatelet, 81% | Aspirin only, 85% | - |
| Follow-up, patient-y | 150 | 108 | 265 | 2645 |
| Mortality per 100 patient-y | 1.7 vs 15.6; HR = 0.11* | - | 7.5 | 8.3 vs 11.6; HR = 0.72* |
| Ischemic stroke per 100 patient-y | 1.7 vs 8.1; HR = 0.21 | 2 | 2.3; RRR, 61% | 2.0 vs 3.2; HR = 0.62 |
| ICH per 100 patient-y | 0.8 vs 9.5; HR = 0.10* | 2 | 1.4 | 0.1 vs 0.5; HR = 0.20 |
| Major bleeding per 100 patient-y | 3.5 vs 9.5; HR = 0.28* | - | 3.8; RRR, 57% | 3.6 vs 4.6; HR = 0.80 |
| Net benefit per 100 patient-y | 5.3 vs 36.7; HR = 0.16* | - | - | 8.1 vs 10.9; HR = 0.76* |
ICH, intracranial hemorrhage; HR, hazard ratio; LAA, left atrial appendage, OAC(D), direct oral anticoagulants; RRR, relative risk reduction (relative to the predicted value according to the scales); SMT, standard medical treatment.
*P <.05.
In all these studies, a common feature is the absence of procedure- or device-related deaths. Evidence in support of reductions in the incidence of complications has already been shown in device-use registries, such as EWOLUTION5, which reported implantation success rates of more than 98% and major complications related to the procedure or device of less than 3%. Even in groups with less experience, there are very acceptable complication rates, which are probably due to widespread dissemination of knowledge of the technique and shorter learning periods. It seems then that one of the justifications for retaining the IIb indication in the guidelines is weakening. The program developed by the Spanish Health Ministry to monitor the results of this technique should help clarify doubts on this issue (Figure 1).
In the very near future, more information will be provided by studies comparing percutaneous LAA occlusion with the new direct OACs. These studies include: Evaluation of WATCHMAN Left Atrial Appendage Occlusion Device in Patients With Atrial Fibrillation Versus Rivaroxaban (NCT02549963); PRAGUE-17: Left Atrial Appendage Closure vs. Novel Anticoagulation Agents in Atrial Fibrillation (NCT02426944); A Pilot Study of Edoxaban in Patients With Non-Valvular Atrial Fibrillation and Left Atrial Appendage Closure (NCT03088072); Safety and Efficacy of Left Atrial Appendage Closure Versus Antithrombotic Therapy in Patients With Atrial Fibrillation Undergoing Drug-Eluting Stent Implantation Due to Complex Coronary Artery Disease (NCT02606552); and Prevention of Stroke by Left Atrial Appendage Closure in Atrial Fibrillation Patients After Intracerebral Hemorrhage (NCT02830152).
These studies will also increase knowledge in another area in which LAA occlusion has been shown to be superior to OAC therapy: that is, cost-effectiveness analysis. Previous studies6 have shown that LAA occlusion is superior (ie, the most effective and least costly) to direct OAC therapy at 5 years and warfarin therapy at 10 years.
In conclusion, percutaneous LAA occlusion is a well-established therapy for highly complex patients (previous bleeding, intracranial hemorrhage) in whom it is difficult to apply OAC therapy. We look forward to the results of comparisons between this technique and the new direct OACs. These results should fill in knowledge gaps and encourage the grade of recommendation of this therapy to be upgraded in the next update of the AF guidelines.
CONFLICTS OF INTERESTA Pérez de Prado is a proctor in LAA occlusion procedures for Boston Scientific. M Pan has been a scientific speaker for Abbott Vascular.
.