Magnetic resonance imaging (MRI) is the technique of choice for soft tissue characterization. Interest in performing MRIs in patients with a pacemaker (PM) or implantable cardioverter-defibrillator (ICD) has increased in the past few years. Despite being contraindicated until recently, it now appears unreasonable to deprive these patients of an investigation that will be indicated in an estimated 75% at some point during their life.1,2
The 2013 European Society of Cardiology Guidelines on cardiac pacing and cardiac resynchronization therapy 2 has a flowchart with safety precautions for performing MRI in patients with conventional PMs and ICDs based on pooled evidence, and recommends following manufacturers’ instructions for PMs and ICDs that are compatible with MRI (MR-conditional devices), which have been available since 2008. Systems labeled “MR-conditional” are those approved by competent authorities after demonstrating an acceptably low risk of complications when the generator and leads are tested together in a defined MR environment.
Some interesting articles have been published on this subject in the past year, 4 of which are of particular interest.
The MagnaSafe Registry3 was a prospective, multicenter study that analyzed the risk of performing nonthoracic MRI at 1.5 tesla for patients with conventional PMs (1000 patients, of whom 284 were pacing dependent) and ICDs (500 patients), excluding pacing-dependent patients with an ICD. No deaths, device failures, losses of capture, need for generator or lead replacement, or ventricular arrhythmias occurred during the MRI. A self-terminating atrial fibrillation episode was registered in 6 cases, and partial generator electrical reset was also observed in 6 cases. One ICD generator, which had not been appropriately programmed pre-MRI, required immediate replacement. Repeat MRI was not associated with adverse events. An MRI was performed within 90 days of device implant in 46 patients with a PM and in 17 with an ICD. Clinical correlation between wave change variables and implantation time was not found.
Two consensus papers have reviewed publications to date. One was authored by the German cardiology and radiology societies,4 and the other was developed by the Heart Rhythm Society (HRS) in collaboration with 11 US, Japanese and European societies for arrhythmias, cardiology, oncology, and radiology.5 The authors of both papers conducted a detailed analysis of the physical and pathophysiological factors for the potential risks of MRI among patients with a PM or ICD, and they established highly specific MRI protocols to minimize existing risks. Both papers describe the responsibilities of cardiologists, radiologists and referring physicians in an MR procedure, stressing the need to adapt to individual cases, weighing up the risk-benefit, and being duly prepared to solve complications should they arise.
Unlike the European Guidelines, the German paper defines the presence of abandoned leads as a relative rather than an absolute contraindication in justified cases in nonpacing-dependent patients requiring nonthoracic MRI. The HRS consensus statement notes that pooled evidence is still insufficient in this respect and it contraindicates MRI in these cases. In view of the MagnaSafe Registry results,3 MRI can be performed in patients with recently implanted devices if clinically required.
Both papers stress that MRI is not risk free in patients with a PM or ICD, including MR-conditional devices, and patients should therefore be informed of these risks. Institutions should follow the expert recommendations and also adapt protocols to their own context.
In any case, it is always recommended to assess the following factors: need for the MRI, patient's electrophysiological risk (PM dependence, arrhythmic risk), type and condition of the device and leads (PM, ICD, MR-conditional, pacing and sensing thresholds, battery status, presence of abandoned, epicardial leads, etc.) pre-MRI interrogation/programming, MR system characteristics, monitoring during the MRI (continuous pulse oximetry, ECG), immediate post-MRI interrogation/programming, cardiopulmonary resuscitation equipment, expert personnel and device programmer present during MRI, and device check-up 3 months after the MRI.
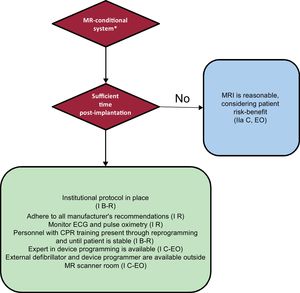
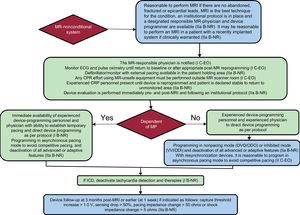
Flowcharts for MR-conditional and conventional devices recommended by the HRS consensus are shown in Figure 1 and Figure 2. The same consensus provides a checklist with all the items that should be taken into consideration when assessing MRI safety.
Protocol for performing MRI in patients with MR-conditional devices. In parenthesis, class of recommendation and level of evidence. CPR, cardiopulmonary resuscitation; EO, expert opinion; R, randomized studies; MRI, magnetic resonance imaging. * An MR-conditional system requires the MR-conditional device and leads to be made by the same manufacturer and for there to be no other implanted element.
Protocol for performing MRI in patients with MR-nonconditional devices. In parenthesis, class of recommendation and level of evidence. CPR, cardiopulmonary resuscitation; EO, expert opinion; ICD, implantable cardioverter-defibrillator; R, randomized studies; MRI, magnetic resonance imaging; NR, nonrandomized studies; PM, pacemaker.
Finally, the cardiovascular and radiology departments of the University of Michigan published the results of 160 MRIs (95 cardiac MRIs) in 36 patients with a PM and 106 with ICD excluded from published protocols (46 had abandoned leads, 19 had an ICD and were PM-dependent, 1 had a recently implanted PM, 2 had battery depletion, and 32 had devices or components on advisory).6 There were no major complications, and although some device parameters changed slightly, they required no reprogramming. In one patient who had an ICD on advisory, the pacing rate fell substantially. The authors concluded that MRI can be performed safely in this type of patient, provided a specific protocol is followed.
.