Aortic self-expandable (SE) transcatheter aortic valve implantation (TAVI) devices are particularly useful for patients with aortic stenosis and small/tortuous vessels, small aortic annuli, or low coronary ostia. However, it is unclear whether the growing range of SE devices shows comparable hemodynamic and clinical outcomes. We aimed to determine the differential hemodynamic (residual valve area and regurgitation) and clinical outcomes of these devices in comparable scenarios.
MethodsAll patients were enrolled from 4 institutions and were managed with 4 different SE TAVI devices. Baseline and follow-up clinical data were collected and echocardiographic tests blindly and centrally analyzed. Patients were compared according to valve type and a 1:1 matched comparison was performed according to degree of calcification, aortic annulus dimensions, left ventricular ejection fraction, and body surface area.
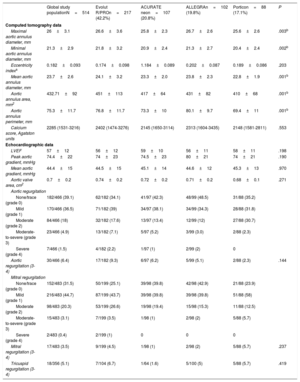
ResultsIn total, 514 patients were included (Evolut R/PRO, 217; ACURATE neo, 107; ALLEGRA, 102; Portico, 88). Surgical risk scores were comparable in the unmatched population. No differences were observed in the post-TAVI regurgitation rate and in in-hospital mortality (2.7%). The rate of pacemaker implantation at discharge was significantly different among devices (P=.049), with Portico showing the highest rate (23%) and ACURATE neo the lowest (9.5%); Evolut R/PRO and ALLEGRA had rates of 15.9% and 21.2%, respectively. The adjusted comparison showed worse residual TAVI gradients and aortic valve area with ACURATE neo vs ALLEGRA (P=.001) but the latter had higher risk of valve embolization and a tendency for more cerebrovascular events.
ConclusionsA matched comparison of 4 SE TAVI devices showed no differences regarding residual aortic regurgitation and in-hospital mortality.
Keywords
Transcatheter aortic valve implantation (TAVI) is a prominent therapeutic alternative for patients with severe aortic stenosis at high, intermediate, or low surgical risk.1–4 The design of the most widespread commercially available percutaneous devices includes balloon- and self-expandable (SE) prostheses. In particular, SE valves are the preferred alternative in certain scenarios, including those involving a high degree of calcification,5 small annuli,6 low coronary ostia,7 marked tortuosity of the ascending aorta,8 nontransfemoral transvascular access,9 and a smaller vessel size. However, SE devices have been linked to a higher rate of moderate-to-severe paravalvular leak (PVL) that might result in up to a 3-fold increase in mortality at 1-year follow-up.10,11 Although postdilatation of the prosthesis might reduce the incidence of PVL, this approach is not without risks because it has been associated with a higher rate of new conduction disorders (which are also a major concern with most SE devices), valve migration, annular rupture, and stroke.12
Several measures have been implemented to reduce these complications, including adoption of sealing skirts, more homogeneous radial force expansion, and repositionable properties.13 It remains unclear if the growing range of newer iteration SE devices behaves equally in terms of hemodynamics, conduction abnormalities, and clinical outcomes. Accordingly, we compared the main clinical outcomes and hemodynamic performance—as assessed through blinded central echocardiographic analysis—of the 4 SE devices available in our setting: Evolut R/PRO (Medtronic, United States), ACURATE neo (Boston Scientific, United States), ALLEGRA (New Valve Technology AG, Switzerland), and Portico (Abbott, United States).
METHODSStudy populationThis retrospective study included 514 consecutive symptomatic patients with severe aortic stenosis of the native valve who received SE TAVI devices in any of 4 centers. The data and images of all procedures performed between January 2017 and January 2019 were collected in a dedicated database after signed informed consent was provided by the patients and approval obtained from the local ethics committees.
In all cases, the Heart Team of each institution determined patient suitability and eligibility for the procedure and the valve type. To be included in the study, patients were required to have baseline, in-hospital, and 30-day echocardiographic images. Also required were multidetector computed tomography data and the main clinical, procedural, and long-term outcomes.
The primary endpoint was valve hemodynamic performance based on echocardiographic parameters, and therefore matched comparisons were performed by matching alternative pairs of devices. Secondary endpoints were based on the VARC-2 consensus and included cardiovascular mortality, myocardial infarction, cerebrovascular events, bleeding complications, acute kidney injury, vascular complications, conduction abnormalities and arrhythmia, repeat hospitalization, and New York Heart Association (NYHA) functional class.
Imaging analysisEchocardiographic examinations were performed according to the guidelines of the American Society of Echocardiography before the procedure, at discharge, and at 30-day follow-up. The following measurements were obtained: left ventricular outflow tract diameter, left ventricular ejection fraction using the biplane Simpson method, mean and peak transvalvular gradients, area by continuity equation, and the presence, degree, and type (transvalvular, paravalvular, global) of aortic regurgitation (AR). AR severity was evaluated using a multiparametric approach and classified following VARC-2 recommendations14 as follows: 0, none/trace; 1, mild; 2, mild-to-moderate; 3, moderate; and 4, severe. Grades 3 and 4 were considered significant AR. Location and circumferential extent were also assessed for paravalvular AR. The circumferential extent of the paravalvular jets was measured in parasternal short-axis views using color Doppler imaging.15 Images were centrally analyzed16 by 2 independent operators (SVV and SSM) blinded to the type of prosthesis and a lack of significant differences was assessed in 10% of the studies. The initial quality of the images was assessed to determine the proportion of patients with information on the main imaging endpoints, including baseline left ventricular ejection fraction (available in 91.6%), peak and mean aortic gradients (92.8%), estimated aortic valve area (83.3%), presence and global degree of AR (90.6%), mitral regurgitation degree (93.6%), and tricuspid regurgitation (69.2%). At the 30-day follow-up, left ventricular ejection fraction was available in 91.6% of the studies, peak and mean aortic gradient in 88%, aortic valve area in 81.5%, indexed aortic valve area in 81.3%, and the presence, global degree, and location of AR—peri- or intraprosthetic—in 99.2%.
Multidetector computed tomography examinations were performed according to the guidelines of the Society of Cardiovascular Computed Tomography17 and good-quality examinations were available in 479 patients (93.5% of the global study population). The main parameters were aortic annulus dimensions (diameters, perimeter, and area), perimeter and area-derived diameters, eccentricity index, and aortic valve calcification graded according to the calcium score (Agatston units).
Statistical analysisCategorical variables are presented as frequencies, and comparisons between groups were performed using the chi-square or Fisher exact test. Continuous variables are expressed as mean (± standard deviation) or median [25th-75th interquartile range] and were analyzed for normal distribution with the Kolmogorov-Smirnov test. Comparisons between groups were performed using the t test or Mann-Whitney U test according to variable distribution. ANOVA was used for comparisons between multiple groups. Differences were considered statistically significant at P<.05.
Seven propensity scores were used for a 1-to-1 comparison of the 4 types of valves and included the following variables: left ventricular ejection fraction (within 10%, as assessed by transthoracic echocardiography), aortic annulus diameter (within 0.5mm) and area (within 50 mm2) (measured using computed tomography), body surface area (within 0.4 m2), body mass index (within 5kg/m2), and degree of calcification (within 500 AU), despite the lack of baseline differences. Pairs of patients were derived using the greedy nearest neighbor method 1:1 with one-fifth of the standard deviation of the logit of the propensity score as caliper with the MatchIt package.18 After matching, comparisons between groups were performed using McNemar test for categorical variables and paired t test for continuous variables. Kaplan-Meier analysis was performed using the log-rank test to compare survival rates between groups.
All analyses were conducted using IBM SPSS Statistics version 24 (IBM, United States).
RESULTSOf a total of 826 patients who underwent TAVI within the study period, 514 (62.2%) received 1 of the 4 SE devices: 42.2% (n=217) received an Evolut R/PRO valve, 20.8% (n=107) an ACURATE neo valve, 19.8% (n=102) an ALLEGRA Valve, and 17.1% (n=88) a Portico valve. All 4 participating institutions used the 4 devices compared in this research and did not show significant differences in main outcomes.
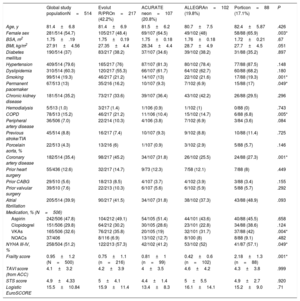
Baseline clinical and imaging characteristicsThe main baseline characteristics of the patients are summarized in table 1. The mean age of the population was 81.4±6.8 years and 54.7% were women; higher proportion of women were treated with ACURATE neo and Portico valves (64.5% and 65.9%, respectively; P=.003). At baseline, more patients who were deemed candidates for Evolut R/PRO (16.2%) and Portico (17%) had a permanent pacemaker vs less than 10% of those treated with alternative SE devices (P=.049). Regarding the baseline risk, no differences were found in the STS score or EuroSCORE, but patients in the Portico group had a higher frailty score (2.18±1.3) than those in the other groups (P=.001).
Main baseline characteristics of the global study population and according to valve type
| Global study populationN=514 | Evolut R/PROn=217 (42.2%) | ACURATE neon=107 (20.8%) | ALLEGRAn=102 (19.8%) | Porticon=88 (17.1%) | P | |
|---|---|---|---|---|---|---|
| Age, y | 81.4±6.8 | 81.4±6.9 | 81.5±6.2 | 80.7±7.5 | 82.4±5.87 | .426 |
| Female sex | 281/514 (54.7) | 105/217 (48.4) | 69/107 (64.5) | 49/102 (48) | 58/88 (65.9) | .003* |
| BSA, m2 | 1.75±.19 | 1.75±0.19 | 1.75±0.18 | 1.76±0.18 | 1.72±0.21 | .67 |
| BMI, kg/m2 | 27.91±4.56 | 27.35±4.4 | 28.34±4.4 | 28.7±4.9 | 27.7±4.5 | .051 |
| Diabetes mellitus | 190/514 (37) | 83/217 (38.2) | 37/107 (34.6) | 39/102 (38.2) | 31/88 (35.2) | .897 |
| Hypertension | 409/514 (79.6) | 165/217 (76) | 87/107 (81.3) | 80/102 (78.4) | 77/88 (87.5) | .148 |
| Dyslipidemia | 310/514 (60.3) | 120/217 (55.3) | 66/107 (61.7) | 64/102 (62.7) | 60/88 (68.2) | .180 |
| Smoking | 99/514 (19.3) | 46/217 (21.2) | 14/107 (13) | 22/102 (21.6) | 17/88 (19.3) | .001* |
| Permanent pacemaker | 67/513 (13) | 35/216 (16.2) | 10/107 (9.3) | 7/102 (6.9) | 15/88 (17) | .049* |
| Chronic kidney disease | 181/514 (35.2) | 73/217 (33.6) | 39/107 (36.4) | 43/102 (42.2) | 26/88 (29.5) | .296 |
| Hemodialysis | 5/513 (1.0) | 3/217 (1.4) | 1/106 (0.9) | 1/102 (1) | 0/88 (0) | .743 |
| COPD | 78/513 (15.2) | 46/217 (21.2) | 11/106 (10.4) | 15/102 (14.7) | 6/88 (6.8) | .005* |
| Peripheral artery disease | 36/506 (7.0) | 22/214 (10.3) | 4/106 (3.8) | 7/102 (6.9) | 3/84 (3.6) | .084 |
| Previous stroke/TIA | 45/514 (8.8) | 16/217 (7.4) | 10/107 (9.3) | 9/102 (8.8) | 10/88 (11.4) | .725 |
| Porcelain aorta, % | 22/513 (4.3) | 13/216 (6) | 1/107 (0.9) | 3/102 (2.9) | 5/88 (5.7) | .146 |
| Coronary artery disease | 182/514 (35.4) | 98/217 (45.2) | 34/107 (31.8) | 26/102 (25.5) | 24/88 (27.3) | .001* |
| Prior heart surgery | 55/436 (12.6) | 32/217 (14.7) | 9/73 (12.3) | 7/58 (12.1) | 7/88 (8) | .449 |
| Prior CABG | 29/510 (5.6) | 18/213 (8.5) | 4/107 (3.7) | 4/102 (3.9) | 3/88 (3.4) | .155 |
| Prior valvular surgery | 39/510 (7.6) | 22/213 (10.3) | 6/107 (5.6) | 6/102 (5.9) | 5/88 (5.7) | .292 |
| Atrial fibrillation | 205/514 (39.9) | 90/217 (41.5) | 34/107 (31.8) | 38/102 (37.3) | 43/88 (48.9) | .093 |
| Medication, % (N=506) | ||||||
| Aspirin | 242/506 (47.8) | 104/212 (49.1) | 54/105 (51.4) | 44/101 (43.6) | 40/88 (45.5) | .658 |
| Clopidogrel | 151/506 (29.8) | 64/212 (30.2) | 30/105 (28.6) | 23/101 (22.8) | 34/88 (38.6) | .124 |
| VKAs | 165/506 (32.6) | 76/212 (35.8) | 20/105 (19) | 32/101 (31.7) | 37/88 (42) | .004* |
| NOACs | 37/406 | 8/116 (6.9) | 13/102 (12.7) | 8/100 (8) | 8/88 (9.1) | .482 |
| NYHA III-IV, % | 258/504 (51.2) | 122/213 (57.3) | 42/102 (41.2) | 53/102 (52) | 41/87 (57.1) | .049* |
| Frailty score | 0.95±1.2 (N=500) | 0.75±1.1 (n=216) | 0.81±1 (n=99) | 0.42±0.6 (n=102) | 2.18±1.3 (n=88) | .001* |
| TAVI score (from ACC) | 4.1±3.2 | 4.2±3.9 | 4±3.5 | 4.6±4.2 | 4.3±3.8 | .999 |
| STS score | 4.9±4.33 | 5±4.1 | 4.4±1.4 | 5±5.5 | 4.9±2.7 | .920 |
| Logistic EuroSCORE | 15.5±10.84 | 15.9±11.4 | 13.4±8.3 | 16.1±14.1 | 15.2±9.0 | .71 |
ACC, American College of Cardiology; BMI, body mass index; BSA, body surface area; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; NOACs, new oral anticoagulants; STS, Society of Thoracic Surgeons; NYHA, New York Heart Association; TAVI, transcatheter aortic valve implantation; TIA, transient ischemic attack; VKAs, vitamin K antagonists.
Data are expressed as No. (%) or mean±standard deviation.
Echocardiographic and multidetector computed tomography findings at baseline according to valve type are presented in table 2. There were no differences between the groups in aortic stenosis severity (P> .50 for mean transvalvular gradient) or in left ventricular ejection fraction. As shown in table 2, the mean size of the aortic annulus as assessed by computed tomography was significantly larger in patients treated with the Evolut R/PRO device, but the degree of calcification was comparable among the device groups.
Main baseline echocardiographic and computed tomography findings of the global study population and according to valve type
| Global study populationN=514 | Evolut R/PROn=217 (42.2%) | ACURATE neon=107 (20.8%) | ALLEGRAn=102 (19.8%) | Porticon=88 (17.1%) | P | |
|---|---|---|---|---|---|---|
| Computed tomography data | ||||||
| Maximal aortic annulus diameter, mm | 26±3.1 | 26.6±3.6 | 25.8±2.3 | 26.7±2.6 | 25.6±2.6 | .003b |
| Minimal aortic annulus diameter, mm | 21.3±2.9 | 21.8±3.2 | 20.9±2.4 | 21.3±2.7 | 20.4±2.4 | .002b |
| Eccentricity indexa | 0.182±0.093 | 0.174±0.098 | 1.184±0.089 | 0.202±0.087 | 0.189±0.086 | .203 |
| Mean aortic annulus diameter, mm | 23.7±2.6 | 24.1±3.2 | 23.3±2.0 | 23.8±2.3 | 22.8±1.9 | .001b |
| Aortic annulus area, mm2 | 432.71±92 | 451±113 | 417±64 | 431±82 | 410±68 | .001b |
| Aortic annulus perimeter, mm | 75.3±11.7 | 76.8±11.7 | 73.3±10 | 80.1±9.7 | 69.4±11 | .001b |
| Calcium score, Agatston units | 2285 (1531-3216) | 2402 (1474-3276) | 2145 (1650-3114) | 2313 (1604-3435) | 2148 (1581-2811) | .553 |
| Echocardiographic data | ||||||
| LVEF | 57±12 | 56±12 | 59±10 | 56±11 | 58±11 | .198 |
| Peak aortic gradient, mmHg | 74.4±22 | 74±23 | 74.5±23 | 80±21 | 74±21 | .190 |
| Mean aortic gradient, mmHg | 44.4±15 | 44.5±15 | 45.1±14 | 44.6±12 | 45.3±13 | .970 |
| Aortic valve area, cm2 | 0.7±0.2 | 0.74±0.2 | 0.72±0.2 | 0.71±0.2 | 0.68±0.1 | .271 |
| Aortic regurgitation | ||||||
| None/trace (grade 0) | 182/466 (39.1) | 62/182 (34.1) | 41/97 (42.3) | 48/99 (48.5) | 31/88 (35.2) | |
| Mild (grade 1) | 170/466 (36.5) | 71/182 (39) | 34/97 (38.1) | 34/99 (34.3) | 28/88 (31.8) | |
| Moderate (grade 2) | 84/466 (18) | 32/182 (17.6) | 13/97 (13.4) | 12/99 (12) | 27/88 (30.7) | |
| Moderate-to-severe (grade 3) | 23/466 (4.9) | 13/182 (7.1) | 5/97 (5.2) | 3/99 (3.0) | 2/88 (2.3) | |
| Severe (grade 4) | 7/466 (1.5) | 4/182 (2.2) | 1/97 (1) | 2/99 (2) | 0 | |
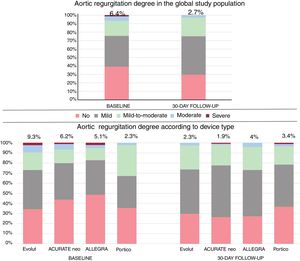
| Aortic regurgitation (3-4) | 30/466 (6.4) | 17/182 (9.3) | 6/97 (6.2) | 5/99 (5.1) | 2/88 (2.3) | .144 |
| Mitral regurgitation | ||||||
| None/trace (grade 0) | 152/483 (31.5) | 50/199 (25.1) | 39/98 (39.8) | 42/98 (42.9) | 21/88 (23.9) | |
| Mild (grade 1) | 216/483 (44.7) | 87/199 (43.7) | 39/98 (39.8) | 39/98 (39.8) | 51/88 (58) | |
| Moderate (grade 2) | 98/483 (20.3) | 53/199 (26.6) | 19/98 (19.4) | 15/98 (15.3) | 11/88 (12.5) | |
| Moderate-to-severe (grade 3) | 15/483 (3.1) | 7/199 (3.5) | 1/98 (1) | 2/98 (2) | 5/88 (5.7) | |
| Severe (grade 4) | 2/483 (0.4) | 2/199 (1) | 0 | 0 | 0 | |
| Mitral regurgitation (3-4) | 17/483 (3.5) | 9/199 (4.5) | 1/98 (1) | 2/98 (2) | 5/88 (5.7) | .237 |
| Tricuspid regurgitation (3-4) | 18/356 (5.1) | 7/104 (6.7) | 1/64 (1.6) | 5/100 (5) | 5/88 (5.7) | .419 |
LVEF, left ventricular ejection fraction. Data are expressed as no./N (%) or mean ± standard deviation.
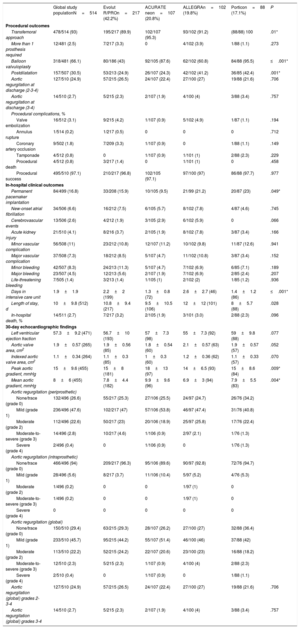
The main procedural and in-hospital events after TAVI are summarized in table 3. The transfemoral approach was the most common route of implantation (93%) in all groups, followed by a transsubclavian approach. Although there were no differences in the preimplantation invasive mean aortic gradient, it was significantly lower with the ALLEGRA valve than with the other devices (P=.049). The rate of predilatation varied widely among devices, from 43% with Evolut R/PRO to 95.5% with Portico (P ≤.001). In addition, postdilatation was less common after Evolut R/PRO (24.9%) and ACURATE neo (24.3%) than after ALLEGRA (41.2%) and Portico (42.4%) (P=.001). In the unmatched population, no differences were found in the degree of AR after valve implantation. Major and minor vascular complications were similar in all of the groups and no significant differences were observed.
Main procedural and in-hospital outcomes of the global study population and according to valve type
| Global study populationN=514 | Evolut R/PROn=217 (42.2%) | ACURATE neon=107 (20.8%) | ALLEGRAn=102 (19.8%) | Porticon=88 (17.1%) | P | |
|---|---|---|---|---|---|---|
| Procedural outcomes | ||||||
| Transfemoral approach | 478/514 (93) | 195/217 (89.9) | 102/107 (95.3) | 93/102 (91.2) | (88/88) 100 | .01* |
| More than 1 prosthesis required | 12/481 (2.5) | 7/217 (3.3) | 0 | 4/102 (3.9) | 1/88 (1.1) | .273 |
| Balloon valvuloplasty | 318/481 (66.1) | 80/186 (43) | 92/105 (87.6) | 62/102 (60.8) | 84/88 (95.5) | ≤.001* |
| Postdilatation | 157/507 (30.5) | 53/213 (24.9) | 26/107 (24.3) | 42/102 (41.2) | 36/85 (42.4) | .001* |
| Aortic regurgitation at discharge (2-3-4) | 127/510 (24.9) | 57/215 (26.5) | 24/107 (22.4) | 27/100 (27) | 19/88 (21.6) | .706 |
| Aortic regurgitation at discharge (3-4) | 14/510 (2.7) | 5/215 (2.3) | 2/107 (1.9) | 4/100 (4) | 3/88 (3.4) | .757 |
| Procedural complications, % | ||||||
| Valve embolization | 16/512 (3.1) | 9/215 (4.2) | 1/107 (0.9) | 5/102 (4.9) | 1/87 (1.1) | .194 |
| Annulus rupture | 1/514 (0.2) | 1/217 (0.5) | 0 | 0 | 0 | .712 |
| Coronary artery occlusion | 9/502 (1.8) | 7/209 (3.3) | 1/107 (0.9) | 0 | 1/88 (1.1) | .149 |
| Tamponade | 4/512 (0.8) | 0 | 1/107 (0.9) | 1/101 (1) | 2/88 (2.3) | .229 |
| Procedural death | 4/512 (0.8) | 3/217 (1.4) | 0 | 1/101 (1) | 0 | .458 |
| Procedural success | 495/510 (97.1) | 210/217 (96.8) | 102/105 (97.1) | 97/100 (97) | 86/88 (97.7) | .977 |
| In-hospital clinical outcomes | ||||||
| Permanent pacemaker implantation | 84/499 (16.8) | 33/208 (15.9) | 10/105 (9.5) | 21/99 (21.2) | 20/87 (23) | .049* |
| New-onset atrial fibrillation | 34/506 (6.6) | 16/212 (7.5) | 6/105 (5.7) | 8/102 (7.8) | 4/87 (4.6) | .745 |
| Cerebrovascular events | 13/506 (2.6) | 4/212 (1.9) | 3/105 (2.9) | 6/102 (5.9) | 0 | .066 |
| Acute kidney injury | 21/510 (4.1) | 8/216 (3.7) | 2/105 (1.9) | 8/102 (7.8) | 3/87 (3.4) | .166 |
| Minor vascular complication | 56/508 (11) | 23/212 (10.8) | 12/107 (11.2) | 10/102 (9.8) | 11/87 (12.6) | .941 |
| Major vascular complication | 37/508 (7.3) | 18/212 (8.5) | 5/107 (4.7) | 11/102 (10.8) | 3/87 (3.4) | .152 |
| Minor bleeding | 42/507 (8.3) | 24/213 (11.3) | 5/107 (4.7) | 7/102 (6.9) | 6/85 (7.1) | .189 |
| Major bleeding | 23/507 (4.5) | 12/213 (5.6) | 2/107 (1.9) | 7/102 (6.9) | 2/85 (2.4) | .207 |
| Life-threatening bleeding | 7/505 (1.4) | 3/213 (1.4) | 1/105 (1) | 2/102 (2) | 1/85 (1.2) | .936 |
| Days in intensive care unit | 1.9±1.9 | 2.2±2 (199) | 1.3±0.8 (72) | 2.6±2.7 (46) | 1.4±1.2 (86) | ≤.001* |
| Length of stay, d | 10±9.8 (512) | 10.8±9.4 (217) | 9.5±10.5 (106) | 12±12 (101) | 8±5.7 (88) | .028 |
| In-hospital death, % | 14/511 (2.7) | 7/217 (3.2) | 2/105 (1.9) | 3/101 (3.0) | 2/88 (2.3) | .096 |
| 30-day echocardiographic findings | ||||||
| Left ventricular ejection fraction | 57.3±9.2 (471) | 56.7±10 (193) | 57±7.3 (98) | 55±7.3 (92) | 59±9.8 (88) | .077 |
| Aortic valve area, cm2 | 1.9±0.57 (265) | 1.9±0.56 (85) | 1.8±0.54 (60) | 2.1±0.57 (63) | 1.9±0.57 (57) | .052 |
| Indexed aortic valve area, cm2 | 1.1±0.34 (264) | 1.1±0.3 (85) | 1±0.3 (60) | 1.2±0.36 (62) | 1.1±0.33 (57) | .070 |
| Peak aortic gradient, mmHg | 15±9.6 (455) | 15±8 (181) | 18±13 (97) | 14±6.5 (93) | 15±8.6 (84) | .009* |
| Mean aortic gradient, mmHg | 8±6 (455) | 7.8±4.4 (182) | 9.9±9.6 (96) | 6.9±3 (94) | 7.9±5.5 (83) | .004* |
| Aortic regurgitation (periprosthetic) | ||||||
| None/trace (grade 0) | 132/496 (26.6) | 55/217 (25.3) | 27/106 (25.5) | 24/97 (24.7) | 26/76 (34.2) | |
| Mild (grade 1) | 236/496 (47.6) | 102/217 (47) | 57/106 (53.8) | 46/97 (47.4) | 31/76 (40.8) | |
| Moderate (grade 2) | 112/496 (22.6) | 50/217 (23) | 20/106 (18.9) | 25/97 (25.8) | 17/76 (22.4) | |
| Moderate-to-severe (grade 3) | 14/496 (2.8) | 10/217 (4.6) | 1/106 (0.9) | 2/97 (2.1) | 1/76 (1.3) | |
| Severe (grade 4) | 2/496 (0.4) | 0 | 1/106 (0.9) | 0 | 1/76 (1.3) | |
| Aortic regurgitation (intraprosthetic) | ||||||
| None/trace (grade 0) | 466/496 (94) | 209/217 (96.3) | 95/106 (89.6) | 90/97 (92.8) | 72/76 (94.7) | |
| Mild (grade 1) | 28/496 (5.6) | 8/217 (3.7) | 11/106 (10.4) | 5/97 (5.2) | 4/76 (5.3) | |
| Moderate (grade 2) | 1/496 (0.2) | 0 | 0 | 1/97 (1) | 0 | |
| Moderate-to-severe (grade 3) | 1/496 (0.2) | 0 | 0 | 1/97 (1) | 0 | |
| Severe (grade 4) | 0 | 0 | 0 | 0 | 0 | |
| Aortic regurgitation (global) | ||||||
| None/trace (grade 0) | 150/510 (29.4) | 63/215 (29.3) | 28/107 (26.2) | 27/100 (27) | 32/88 (36.4) | |
| Mild (grade 1) | 233/510 (45.7) | 95/215 (44.2) | 55/107 (51.4) | 46/100 (46) | 37/88 (42) | |
| Moderate (grade 2) | 113/510 (22.2) | 52/215 (24.2) | 22/107 (20.6) | 23/100 (23) | 16/88 (18.2) | |
| Moderate-to-severe (grade 3) | 12/510 (2.3) | 5/215 (2.3) | 1/107 (0.9) | 4/100 (4) | 2/88 (2.3) | |
| Severe (grade 4) | 2/510 (0.4) | 0 | 1/107 (0.9) | 0 | 1/88 (1.1) | |
| Aortic regurgitation (global) grades 2-3-4 | 127/510 (24.9) | 57/215 (26.5) | 24/107 (22.4) | 27/100 (27) | 19/88 (21.6) | .706 |
| Aortic regurgitation (global) grades 3-4 | 14/510 (2.7) | 5/215 (2.3) | 2/107 (1.9) | 4/100 (4) | 3/88 (3.4) | .757 |
Data are expressed as no./N (%) or mean ± standard deviation.
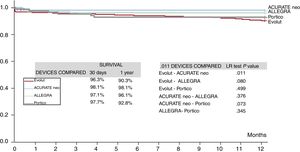
The rate of pacemaker implantation at discharge was significantly different among the device groups (global P=.049), with Portico showing the highest rate (23%) and ACURATE neo the lowest (9.5%); Evolut R/PRO and ALLEGRA had rates of 15.9% and 21.2%, respectively. The need for permanent pacemaker implantation was not related to the degree of aortic valve calcification because there were no differences in calcification among the device groups, even before matching. No differences were found in 30-day mortality among the groups (P=.096), with a global rate of 2.7%, but unadjusted 1-year mortality (6.4%) differed significantly for patients treated with each device: 9.7% for Evolut R/PRO, 1.9% for ACURATE neo, 3.9% for ALLEGRA, and 6.8% for Portico (P=.035), as shown in the survival curves depicted in figure 1. A similar trend was found for 1-year cardiovascular mortality: 4.7% for Evolut R/PRO, 0.7% for ACURATE neo, 1.9% for ALLEGRA, and 3.8% for Portico (P=.079). The main factors associated with 1-year mortality are summarized in and included prior hemodialysis, previous atrial fibrillation, worse baseline NYHA class, valve embolization, cardiac tamponade, and residual moderate or severe AR. The need for permanent pacemaker was not associated with higher mortality.
The specific rates of main complications in patients treated with each device are summarized in table 4, and procedural and in-hospital outcomes in the matched population are specifically reported in . Briefly, the ALLEGRA valve had a better transvalvular mean gradient than ACURATE neo, Evolut, and Portico but the absolute rate of valve embolization was higher with ALLEGRA than with the other valves and this device was associated with more cerebrovascular events vs Portico (P=.032) and Evolut (P=.083).
Main clinical and hemodynamic outcomes between different self-expandable TAVI devices in the unmatched population
| Main characteristics | Evolut/ACURATEN=217/107 | Evolut/ALLEGRAN=217/102 | Evolut/PorticoN=217/88 | ACURATE/ALLEGRAN=107/102 | ACURATE/PorticoN=107/88 | ALLEGRA/PorticoN=102/88 |
|---|---|---|---|---|---|---|
| AR ≥ 3 | 2.3%/1.9%P= .999 | 2.3%/4.0%P=.472 | 2.3%/3.4%P=.695 | 1.9%/4.0%P=.432 | 1.9%/3.4%P=.659 | 4.0%/3.4%P= .999 |
| AR ≥ 2 | 26.5%/22.4%P=.427 | 26.5%/27.0%P=.927 | 26.5%/21.6%P=.370 | 22.4%/27.0%P=.446 | 22.4%/21.6%P=.888 | 27.0%/21.6%P=.389 |
| Mean aortic gradient at discharge | 7.8±4.4/9.9±9.7P=.041* | 7.8±4.4/6.9±3.1P=.083 | 7.8±4.4/7.9±5.5P=.846 | 9.9±9.7/6.9±3.1P=.004* | 9.9±9.7/7.9±5.5P=.093 | 6.9±3.1/7.9±5.5P=.142 |
| Permanent pacemaker implantation | 15.9%/9.5%P=.124 | 15.9%/21.2%P=.250 | 15.9%/23%P=.146 | 9.5%/21.2%P=.020* | 9.5%/23%P=.011* | 21.2%/23%P=.771 |
| Valve embolization | 4.5%/0.9%P=.113 | 4.5%/4.9%P=.772 | 4.5%/1.1%P=.182 | 0.9%/4.9%P=.086 | 0.9%/1.1%P=.883 | 4.9%/1.1%P=.142 |
| Cerebrovascular event | 1.9%/2.9%P=.689 | 1.9%/5.9%P=.083 | 1.9%/0%P=.326 | 2.9%/5.9%P=.253 | 2.9%/3.4%P= .999 | 5.9%/0%P=.032* |
| In-hospital mortality | 3.2%/1.9%P=.723 | 3.2%/3.0%P= .999 | 3.2%/2.3%P= .999 | 1.9%/3.0%P=.678 | 1.9%/2.3%P= .999 | 3.0%/2.3%P= .999 |
AR, aortic regurgitation; TAVI, transcatheter aortic valve implantation.
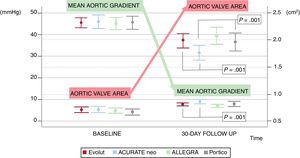
Echocardiographic assessment of the unmatched population is summarized in table 3. A trend to a better valve area and transaortic gradients was found in the ALLEGRA group (figure 2) without significant differences in left ventricular ejection fraction. After matching (table 5), no differences were found in AR presence or degree after valve implantation in any of the pairs (figure 3). However, patients treated with the ACURATE neo valve had a higher mean aortic gradient (8.5±4mmHg) than those treated with the ALLEGRA valve (6.7±2.8, P=.001). Mean gradients and aortic valve areas for each pair are reported in . The center treating the patient was not included in the matched analysis, but univariate analysis showed no impact of this variable on in-hospital mortality (P=.37) or on the rate of AR of any degree (P=.54).
Hemodynamic outcomes and need for permanent pacemaker implantation among different pairs of self-expandable TAVI devices after matchinga
| Evolut | ACURATE neo | Portico | |
|---|---|---|---|
| ACURATE neo | 72 PAIRSAR ≥ 3: 1.4% vs 1.4%, P= .999AR ≥ 2: 22.5% vs 23.9%, P= .999MeanGrdt.: 8.4±5.5 vs 8.3±4.3, P=.926Pacemaker rate: 13.2% vs 5.9%, P=.267 | ||
| Portico | 56 PAIRSAR ≥ 3: 1.8% vs 1.8%, P= .999AR ≥ 2: 23.6% vs 23.6%, P= .999MeanGrdt.: 8.2±6.1 vs 7±3.8, P=.299Pacemaker rate: 11.5% vs 26.9%, P=.096 | 71 PAIRSAR ≥ 3: 1.4% vs 2.8%, P= .999AR ≥ 2: 19.7% vs 21.1%, P= .999MeanGrdt.: 8.8±5 vs 7.5±5, P=.151Pacemaker rate: 7% vs 25.4%, P=.007b | |
| ALLEGRA | 65 PAIRSAR ≥ 3: 3.1% vs 6.3%, P=.687AR ≥ 2: 20.3% vs 23.4%, P=.839MeanGrdt.: 7.7±4.3 vs 6.7±2.9, P=.130Pacemaker rate: 15.3% vs 15.3%, P= .999 | 74 PAIRSAR ≥ 3: 1.4% vs 5.4%, P=.375AR ≥ 2: 28.4% vs 24.3%, P=.690MeanGrdt.: 8.5±4 vs 6.7±2.8, P=.001bPacemaker rate: 9.9% vs 16.9%, P=.332 | 56 PAIRSAR ≥ 3: 5.4% vs 3.6%, P= .999AR ≥ 2: 32.1% vs 26.6%, P=.690MeanGrdt.: 7.6±4 vs 6.6±2.9, P=.114Pacemaker rate: 22.6% vs 20.8%, P= .999 |
AR, aortic regurgitation; MeanGrdt, mean gradient; TAVI, transcatheter aortic valve implantation.
Results show first the values for devices in the first line and then the values for devices in the first column.
Matched variables included: left ventricular ejection fraction (within 10%, as assessed by transthoracic echocardiography), aortic annulus diameter (within 0.5mm) and area (within 50 mm2) (measured using computed tomography), body surface area (within 0.4 m2), body mass index (within 5kg/m2), and the degree of calcification (within 500 Agatston units, measured using computed tomography).
Degree of aortic regurgitation after valve implantation in the global study population and according to valve type in the unmatched population. The percentage of moderate or severe postprocedural aortic regurgitation is presented for the global population and for patients treated with each device.
Although recent comparisons of balloon- and self-expandable devices have reported better global outcomes, mainly driven by a lower PVL rate with balloon-expandable devices,19–22 the current clinical practice does not follow this “all-comers” schema, but a personalized indication of what seems best for each patient. SE TAVI devices are usually preferred in patients with smaller or more tortuous vessels and specifically when these characteristics lead to a need for transsubclavian access, due to the better profile of their delivery systems. Moreover, they are also more often used in small aortic annuli and when there is higher risk of coronary occlusion, aortic annulus rupture, or valve embolization for any reason. The price to pay seems to be a higher risk of PVL and need for pacemaker implantation.23 However, not all SE devices behave similarly in terms of conduction abnormalities and, in particular, no systematic comparison has examined their critical effect on paravalvular regurgitation and residual gradients. Evolut R/PRO and Portico are partially resheathable, unlike ALLEGRA and ACURATE neo. The Portico valve is the only intra-annular valve, and ACURATE neo is the only device that is released from top to bottom. All of these technical differences might have a major clinical impact on patients’ outcomes.
The main findings of our research were as follows. a) There were no differences in terms of residual AR at 30-day follow-up among the 4 SE devices after a careful central echocardiographic analysis and a matched process that considered anatomical features, with a low global rate (2.7%) of more-than-moderate AR. b) Despite a similar degree of valve calcification and after matched paired analysis, the ACURATE neo valve had a higher mean gradient than the ALLEGRA TAVI—not when compared with the others—but half the permanent pacemaker rate of any other device, which, despite being unrelated to the mortality rate, has important implications for patients and the cost-effectiveness of the intervention. c) Valve embolization occurred more often with the ALLEGRA valve, which might partially explain the tendency for a higher rate of cerebrovascular events. d) Although no adjustment according to baseline risk was performed, procedural and 30-day mortality rates were comparable.
Previous comparisons among self-expandable devicesIn the meta-analysis by Barbanti et al.,24 which compared Sapien-3, Lotus, Portico, JenaValve, ACURATE neo, and Evolut R devices, the 30-day mortality (2.2%) and residual more-than-mild AR (1.6%) were comparable to that reported here. However, the authors also highlighted the unresolved issue of the high need for permanent pacemaker implantation (16.2%). Similar findings were reported in the more recent NEOPRO registry25 (Evolut PrO vs ACURATE neo), except for a much lower and comparable rate of pacemaker implantation (12.8% vs 11.0%, P=.565) that is inconsistent with contemporary reports. Costa et al.26 identified pacemaker rates of 8.3% for SAPIEN 3, 16.7% for Evolut R, and 2.1% for ACURATE neo (P <.05). The same registry reported lower gradients with Evolut R than with ACURATE neo (6.1±2.4mmHg vs 8.4±3.5mmHg, P <.01) but comparable residual AR and mortality. In addition, an Italian registry27 reinforced the lower pacemaker rate after the ACURATE neo valve in a matched comparison with Evolut, Portico, Lotus, and Sapien-3. Regarding the Portico valve, its matched comparison vs Sapien-328 suggested a comparable 30-day mortality and similar (> 20%) need for permanent pacemaker implantation and PVL, but a comparison with Evolut-R29 revealed a lower rate of significant PVL with Portico (0%) than with Evolut R (15.2%) in patients with elliptic annulus (P=.034).
Despite the variability in these important outcomes, at least some comparative studies have been performed among Evolut, Portico, and ACURATE neo devices. In contrast, only case series exist for the ALLEGRA valve.30 The hemodynamic outcomes of this newest device showed a mean gradient of 7.2±3.5mmHg with an effective orifice area of 2.06±0.3 cm2. More-than-mild PVL was present in 5.1% of patients before discharge and the pacemaker implantation rate was 13.5% at 30 days, which is in agreement with our findings. The positive post-TAVI gradients reported with the ALLEGRA valve suggest that the supra-annular leaflet position and the radial strength of this device might be particularly useful for small calcified aortic annuli. However, the current inability to resheath the device increases risk and might offset its benefits because it showed the highest rate of valve embolization in our analysis and a high rate of cerebrovascular events. The underlying mechanisms merit deeper insight in future research.
Clinical implication for patient-specific device selectionWhen a SE device is selected to treat a patient with aortic stenosis, not all options are optimal, and they depend on patients’ characteristics. Partially resheathable devices (Evolut/Portico) should probably be selected for patients with high risk of coronary obstruction. On the other hand, these devices should be avoided in patients with high risk of conduction abnormalities, particularly those with a long life-expectancy, in order to reduce permanent pacemaker need, and ACURATE neo probably represents the best alternative. In contrast, the current generation of the Portico valve exhibits the highest rate of conduction abnormalities, despite its appropriate hemodynamic behavior. The new FlexNav delivery system that has been implemented in the newer iteration of the Portico valve probably increases the stability of the device and likely reduces the rate of permanent pacemaker implantation. Moreover, the development of new implantation techniques as the “cusp overlap view” might alter the current scenario regarding post-TAVI conduction abnormalities.31 Finally, ALLEGRA could be useful for patients undergoing a valve-in-valve procedure with a small bioprosthesis because the valve deployment is usually very stable and it might provide better residual gradients than alternative devices such as ACURATE neo,32 with a low risk of valve embolization.
LimitationsThis is a retrospective study; this limitation was addressed through a prospective collection of the data in a similar database in all institutions and through an anonymized core laboratory echocardiographic analysis. The lower number of patients receiving certain devices may reflect an earlier stage in the learning curve and might have affected outcomes. However, the lack of differences in terms of main clinical outcomes or AR degree suggests a potential “class effect” with all SE devices, with a positive impact on the learning curve of newer SE devices in centers already experienced with alternative SE TAVI devices. On the other hand, the higher 1-year mortality rate shown by the patients treated with Evolut R/PRO probably indicates that these patients have anatomical or clinical conditions—not reflected in surgical risk scores—that affect the mid-term prognosis and that this valve may be the preferred TAVI device in more challenging scenarios, given the greater experience of the participating institutions with this system. The 2 iterations of the Evolut valve were analyzed together and they exhibited no differences in term of outcomes. Finally, the slightly lower number of pairs in the matched comparison limits the power of the analysis but, given the concordant finding with the unmatched sample, still confirms the reduced risk of bias in the global study population.
CONCLUSIONSA matched comparison of 4 SE TAVI devices showed no differences in residual AR or 30-day mortality, with a low rate of significant residual AR (2.7%). ACURATE neo was associated with worse residual transvalvular gradients vs ALLEGRA but offered the lowest rate of permanent pacemaker implantation.
FUNDINGNone to declare.
CONFLICTS OF INTERESTL. Nombela-Franco is proctor for Abbott; R. Moreno is proctor for Boston and NVT; J.A. Baz is proctor for NVT; and I.J. Amat-Santos is proctor for Boston. There are no other conflicts of interest regarding this manuscript.
- -
Aortic self-expandable (SE) transcatheter aortic valve implantation (TAVI) devices are particularly useful for patients with aortic stenosis and small/tortuous vessels, small aortic annuli, or low coronary ostia.
- -
However, the growing range of SE devices raises questions about the comparability of their hemodynamic and clinical outcomes.
- -
A matched comparison of 4 SE TAVI devices showed no differences regarding residual AR and in-hospital mortality.
- -
ACURATE neo was superior in terms of the absolute need for permanent pacemaker implantation.
- -
New iterations of current self-expandable TAVI devices should address the excessive pacemaker rate currently presented by most SE TAVI devices through more accurate positioning without increasing the risk of paravalvular leak.
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.rec.2020.09.014