There is limited data on the serial morphological and functional assessment of paclitaxel-coated balloon treatment using coronary angiography, optical coherence tomography, and fractional flow reserve.
MethodsIn this prospective, single-center observational study, patients with de novo lesions were treated with the paclitaxel-coated balloon. Serial angiographic, optical coherence tomography and fractional flow reserve measurements were performed before and after plain old balloon angioplasty, as well as at 9-month follow-up.
ResultsTwenty patients (21 lesions) were enrolled in this study. The reference vessel diameter was 2.68±0.34mm and late luminal loss was 0.01±0.21mm. The median changes in the minimal lumen area between pre- and postplain old balloon angioplasty, and postplain old balloon angioplasty and follow-up were an increase of 75.2% [interquartile range of 37.2 to 164.7] and 50.0% [interquartile range of 1.1% to 64.5%], respectively. Intimal dissections were seen in all postprocedural optical coherence tomography images, and 66.6% of them were sealed on follow-up optical coherence tomography (median 278 days). The fractional flow reserve distal to the target lesion was 0.71±0.14 predilatation, 0.87±0.04 postdilatation, and 0.83±0.08 at follow-up.
ConclusionsThe paclitaxel-coated balloon restores coronary blood flow by means of plaque modification, causing an increment in minimal lumen area. At 9-month follow-up, coronary flow was sustained and the luminal patency was the result of suppressed luminal narrowing progression from local drug effects on the de novo coronary lesions.
Keywords
Stent implantation has significantly improved percutaneous coronary revascularization over the last 3 decades. Although drug-eluting stent (DES) use has decreased in-stent restenosis rates, DES therapy is limited by delayed healing, late acquired malapposition, and neoatherosclerosis leading to an increased risk of late stent thrombosis and late restenosis.1–3 Furthermore, a caged vessel prevents late lumen enlargement and advantageous vascular remodeling.4 Nonstent-based local drug delivery using paclitaxel-coated balloons (PCB) have emerged as a new alternative clinical treatment by maintaining the anti-proliferative properties of DES.5 In a recent study, small vessel de novo lesions treated with PCB only in unselected patients showed a low rate of target lesion revascularization and major adverse cardiovascular events. The PCB was suggested as an alternative treatment option to DES in small vessels with small reference diameters (≥ 2.0mm and ≤ 2.75mm).6 Although the angiographic and clinical effectiveness of PCB in de novo lesions have been suggested, its exact mechanism of action has not been fully explained. There are few data on the functional and intravascular morphological changes induced by PCB over time in de novo lesions. Therefore, the aim of our study was to gain further insight into the treatment of de novo lesions with PCB, focusing on their short-term and mid-term mechanisms. To achieve this, serial angiographic, fractional flow reserve (FFR), and optical coherence tomography (OCT) were performed before intervention, immediately after intervention, and at 9-month follow-up in de novo lesions treated with PCB.
METHODSThis study aimed to assess the morphological and functional changes induced by PCB in de novo coronary lesions and was conducted as an OCT substudy of a single-center prospective registry.7 The study was approved by the Ethics Committee of Ulsan University Hospital, and all participants provided signed informed consent.
Patient SelectionPatients with stable or unstable angina pectoris, scheduled to undergo elective percutaneous coronary intervention for de novo lesions, were considered eligible. Documented ischemia had to be present. Lesions with a reference vessel diameter between 2.5mm and 3.5mm and lesion length of ≤ 24mm were eligible for participation in this study. Exclusion criteria consisted of heart failure (left ventricular ejection fraction<30%), acute myocardial infarction that was diagnosed as troponin-T elevation, left main artery disease, ostial lesion (impossible to assess with OCT), heavily calcified or thrombotic lesions, life expectancy<1 year, and known renal failure (creatinine>2mg/dL).
Interventional Procedure, Optical Coherence Tomography, and Fractional Flow Reserve Data Acquisition and AnalysisAll patients were treated with aspirin 200mg and clopidogrel 300 to 600mg loading dose before the procedure, and 100 U/Kg of unfractionated heparin was injected intravenously to maintain an activated clotting time ≥ 250 s during the procedure. For the lesion preparation, the patient underwent predilation with an optimal sized balloon based on angiography (balloon-to-vessel ratio of 1.0), shorter than the intended length of PCB with nominal pressure inflation. Application of the PCB was decided by the interventional cardiologist performing the procedure based on the FFR measured after plain old balloon angioplasty (POBA).7 Under angiographic guidance, PCB (SeQuent Please, B. Braun; Melsungen, Germany) sized at 1.0 of balloon-to-vessel ratio was delivered as quickly as possible and inflated for 60seconds with nominal pressure. The use of glycoprotein IIb/IIIa inhibitors during the procedure was at the discretion of the operator.
Coronary angiographies were analyzed using the Cardiovascular Angiography Analysis System (CAAS 5.10, Pie Medical Imaging B.V.; Maastricht, The Netherlands) by an independent investigator who was blinded to clinical data before POBA, after PCB application, and at 9-month follow-up.
Optical coherence tomography was performed based at the operator's discretion, before the procedure, after POBA (just before PCB application) and at 9-months’ follow-up. Fourier-domain OCT (C7XR, LightLab Imaging, Inc.; Westford, Massachusetts, United States) was used with the nonocclusive technique. The catheter was advanced distal to the lesion over a conventional 0.014-inch guidewire, and images were obtained by motorized pullback at 20mm/s during continuous flushing of 20mL of contrast media. Offline OCT analysis was performed by a totally independent investigator (J.N. No) using proprietary software (LLI). After calibration for z-offset, 1 frame per 5 frames was analyzed, discarding images with an intervening side branch.
Fractional flow reserve was measured before, after POBA (before PCB application), and at 9-month follow-up but was not performed for subtotal (99% stenosis) lesions without a clinical indication. After intracoronary nitroglycerine injection of 200μg, FFR was measured using a 0.014-inch coronary pressure wire (PressureWire Certus, St. Jude Medical Systems; Uppsala, Sweden) far distal from the lesion under hyperemic conditions induced by intravenous adenosine infusion (140-180μg/kg/min).
Follow-up and Clinical OutcomeAll patients were scheduled to undergo clinical and angiographic follow-up at 9 months. Serial angiographic data, OCT images and FFR measurements were analyzed. Clinical outcomes were defined according to the Academic Research Consortium criteria.8 Binary restenosis was defined as a diameter stenosis ≥ 50% at angiographic follow-up. Late-luminal loss was defined as the difference in minimal luminal diameter between postprocedure and follow-up images in the same segment (in-segment). All outcomes were adjudicated by a clinical events committee.
Statistical AnalysisAnalyses were performed using SPSS 18.0 (SPSS Inc.; Chicago, Illinois, United States). Continuous variables are presented as the mean±standard deviation or median [interquartile range]. Continuous variables were compared between 2 groups using the paired Student t test or Wilcoxon signed rank test, as appropriate. Categorical variables are presented as counts and percentages and were compared using the chi-square or Fischer exact test, as appropriate. A 2-tailed P value of<.05 was considered statistically significant.
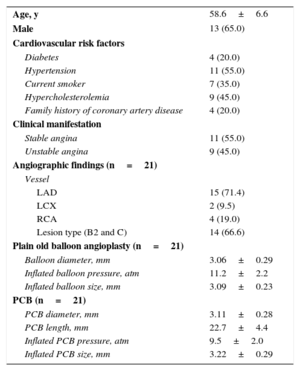
RESULTSPatient and Procedural CharacteristicsBetween June 2012 and June 2013, 20 patients (21 lesions) with OCT images after POBA and at 9 months’ follow-up were included in this study (Figure 1). Baseline clinical and procedural characteristics are shown in Table 1.
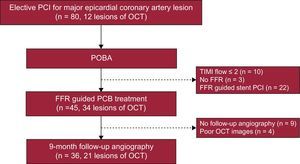
Flow chart of PCB application. Operators decided PCB or stent implantation based on FFR measurement after POBA. Of 45 PCB treated lesions, 21 were included in this OCT sub-study. FFR, fractional flow reserve; OCT, optical computed tomography; PCB, paclitaxel-coated balloon; PCI, percutaneous coronary intervention; POBA, plain old balloon angioplasty; TIMI, Thrombolysis In Myocardial Infarction.
Baseline Characteristics (n=20)
| Age, y | 58.6±6.6 |
| Male | 13 (65.0) |
| Cardiovascular risk factors | |
| Diabetes | 4 (20.0) |
| Hypertension | 11 (55.0) |
| Current smoker | 7 (35.0) |
| Hypercholesterolemia | 9 (45.0) |
| Family history of coronary artery disease | 4 (20.0) |
| Clinical manifestation | |
| Stable angina | 11 (55.0) |
| Unstable angina | 9 (45.0) |
| Angiographic findings (n=21) | |
| Vessel | |
| LAD | 15 (71.4) |
| LCX | 2 (9.5) |
| RCA | 4 (19.0) |
| Lesion type (B2 and C) | 14 (66.6) |
| Plain old balloon angioplasty (n=21) | |
| Balloon diameter, mm | 3.06±0.29 |
| Inflated balloon pressure, atm | 11.2±2.2 |
| Inflated balloon size, mm | 3.09±0.23 |
| PCB (n=21) | |
| PCB diameter, mm | 3.11±0.28 |
| PCB length, mm | 22.7±4.4 |
| Inflated PCB pressure, atm | 9.5±2.0 |
| Inflated PCB size, mm | 3.22±0.29 |
LAD, left anterior descending artery; LCX, left circumflex artery; PCB, paclitaxel-coated balloon; RCA, right coronary artery.
Values are presented as number (%) or mean±standard deviation.
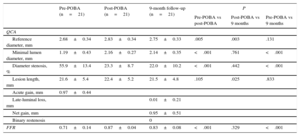
The angiographic quantitative coronary analysis data, FFR and clinical outcomes are presented in Table 2. All patients underwent angiographic follow-up. The reference vessel diameter was 2.68±0.34mm. Late luminal loss and net gain of the lesions were 0.01±0.21mm and 0.95±0.51mm, respectively. Minimal lumen diameter showed no difference between the post-PCB application and a 9 months’ follow-up (2.16±0.27mm vs 2.14±0.35mm; P=.761). There were no angiographic binary restenosis or adverse clinical events except for 1 case of nontarget lesion revascularization.
Serial Quantitative Coronary Angiography and Functional Measurements
| Pre-POBA (n=21) | Post-POBA (n=21) | 9-month follow-up (n=21) | P | |||
|---|---|---|---|---|---|---|
| Pre-POBA vs post-POBA | Post-POBA vs 9 months | Pre-POBA vs 9 months | ||||
| QCA | ||||||
| Reference diameter, mm | 2.68±0.34 | 2.83±0.34 | 2.75±0.33 | .005 | .003 | .131 |
| Minimal lumen diameter, mm | 1.19±0.43 | 2.16±0.27 | 2.14±0.35 | <.001 | .761 | <.001 |
| Diameter stenosis, % | 55.9±13.4 | 23.3±8.7 | 22.0±10.2 | <.001 | .442 | <.001 |
| Lesion length, mm | 21.6±5.4 | 22.4±5.2 | 21.5±4.8 | .105 | .025 | .833 |
| Acute gain, mm | 0.97±0.44 | |||||
| Late-luminal loss, mm | 0.01±0.21 | |||||
| Net gain, mm | 0.95±0.51 | |||||
| Binary restenosis | 0 | |||||
| FFR | 0.71±0.14 | 0.87±0.04 | 0.83±0.08 | <.001 | .329 | <.001 |
QCA, quantitative coronary analysis; POBA, plain old balloon angioplasty; FFR, fractional flow reserve.
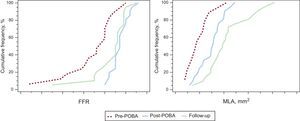
Four vessels had subtotal occlusion (99% stenosis), and FFR was not measured in these vessels. After balloon angioplasty, restored coronary flow was measured as an FFR value of 0.87±0.04 in all enrolled lesions. At 9 months’ follow-up, restored coronary flow was sustained without a significant decrease in FFR measurement (0.83±0.08). Serial FFR results are listed in Table 2 and Figure 2.
Cumulative distribution curves. Serial FFR and MLAs are presented. The post-POBA FFR was not significantly different compared with that at 9 months’ follow-up. There was a significant increment in the MLA from post-POBA to 9 months’ follow-up. FFR, fractional flow reserve; MLA, minimal lumen area; POBA, plain old balloon angioplasty.
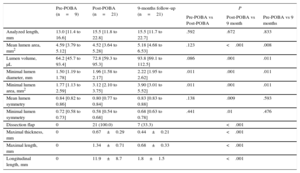
Pre-POBA OCT images were not available in 12 lesions due to the inability to cross the lesions with the OCT catheter or poor contrast filling in total occlusions with the OCT catheter. However, 21 OCT post-POBA and follow-up data were available. Serial OCT findings and the percent changes are presented in Tables 3 and 4. Optical coherence tomography-based mean lumen area and lumen volume increased significantly from post-POBA to follow-up (4.52 vs 5.18mm2, P<.001 and 72.8 vs 93.8mm3, P=.001, respectively). Minimal lumen diameter and minimal lumen area increased significantly after POBA (1.5 vs 1.96mm, P=.001 and 1.77 vs 3.12mm, P=.001, respectively), with a further increase at 9 months (1.96 vs 2.22mm, P=.011 and 3.12 vs 3.90mm, P=.011, respectively). Mean and minimal lumen symmetry did not change between post-POBA and 9-month follow-up, possibly because of the healed dissections of the plaque. The median percent changes of the minimal lumen areas between pre- and post-POBA, and post-POBA and follow-up showed an increase of 75.2% [interquartile range, 37.2% to 164.7%], and 50.0% [interquartile range, 1.1% to 64.5%], respectively. Post-POBA dissections were sealed in 14 lesions (66.7%) at the 9-month follow-up with a decrease in the size of the dissected flaps (Table 3). The maximal thickness and length of residual dissected flaps at the cross-sectional images decreased significantly (0.67±0.29 mm vs 0.44±0.21mm, P<.001 and 1.34±0.71 mm vs 0.68±0.33mm, P<.001, respectively), as shown in Figure 3. The length of dissected flaps on longitudinal images also decreased significantly (11.9±8.7 mm vs 1.8±1.5mm, P<.001). There were no complications related to these procedures.
Serial Optical Coherence Tomography Analysis
| Pre-POBA (n=9) | Post-POBA (n=21) | 9-months follow-up (n=21) | P | |||
|---|---|---|---|---|---|---|
| Pre-POBA vs Post-POBA | Post-POBA vs 9 month | Pre-POBA vs 9 months | ||||
| Analyzed length, mm | 13.0 [11.4 to 16.6] | 15.5 [11.8 to 22.8] | 15.5 [11.7 to 22.7] | .592 | .672 | .833 |
| Mean lumen area, mm2 | 4.59 [3.79 to 5.12] | 4.52 [3.64 to 5.28] | 5.18 [4.68 to 6.53] | .123 | <.001 | .008 |
| Lumen volume, μL | 64.2 [45.7 to 93.4] | 72.8 [59.3 to 95.3] | 93.8 [69.1 to 112.5] | .086 | .001 | .011 |
| Minimal lumen diameter, mm | 1.50 [1.19 to 1.78] | 1.96 [1.58 to 2.17] | 2.22 [1.95 to 2.62] | .011 | .001 | .011 |
| Minimal lumen area, mm2 | 1.77 [1.13 to 2.59] | 3.12 [2.10 to 3.75] | 3.90 [3.01 to 5.52] | .011 | .001 | .011 |
| Mean lumen symmetry | 0.84 [0.82 to 0.86] | 0.80 [0.77 to 0.84] | 0.83 [0.83 to 0.88] | .138 | .009 | .593 |
| Minimal lumen symmetry | 0.72 [0.58 to 0.73] | 0.58 [0.54 to 0.68] | 0.68 [0.63 to 0.78] | .441 | .01 | .476 |
| Dissection flap | 0 | 21 (100.0) | 7 (33.3) | <.001 | ||
| Maximal thickness, mm | 0 | 0.67±0.29 | 0.44±0.21 | <.001 | ||
| Maximal length, mm | 0 | 1.34±0.71 | 0.68±0.33 | <.001 | ||
| Longitudinal length, mm | 0 | 11.9±8.7 | 1.8±1.5 | <.001 | ||
POBA, plain old balloon angioplasty.
Data are expressed as median [interquartile range], No. (%) or mean ± standard deviation.
Lumen symmetry lies between 0 and 1. A value of 1 means fully symmetric, with less symmetry with a decreasing value.
Percentage Changes of Quantitative Coronary Analysis, Optical Coherence Tomography and Fractional Flow Reserve
| Pre-POBA vs post-POBA | Post-POBA vs 9 months | Pre-POBA vs 9 months | |
|---|---|---|---|
| QCA | |||
| Patients | 21 | 21 | 21 |
| Minimal lumen diameter change, % | 75 [55.3 to 142.2] | 1.3 [–7.4 to 4.2] | 79.6 [44.3 to 159.6] |
| Diameter stenosis change, % | –65.3 [–70.2 to –42.6] | –2.9 [–25.3 to 15.6] | –62.0 [76.4 to –37.1] |
| OCT | |||
| Patients | 9 | 21 | 21 |
| Minimal lumen area change, % | 75.2 [37.2 to 164.7] | 50.0 [1.1 to 64.5] | 123.7 [56.5 to 276.9] |
| Mean lumen area change, % | 6.0 [0.5 to 22.5] | 22.8 [5.4 to 39.1] | 31.7 [18.7 to 41.0] |
| FFR | |||
| Patients | 17 | 21 | 21 |
| FFR change, % | 11.3 [5.5 to 21.7] | –1.7 [–10.3 to 2.1] | 7.5 [–0.6 to 22.3] |
FFR, fractional flow reserve; OCT, optical coherence tomography, POBA, plain old balloon angioplasty; QCA, quantitative coronary analysis.
Values are in median [interquartile range].
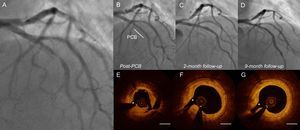
A representative case treated with PCB. A 67-year-old woman had unstable angina, with near total occlusion of the left anterior descending coronary artery (A). She underwent PCB treatment with a 3.0/20mm SeQuent Please (B. Braun; Melsungen, Germany). After treatment, the artery showed minimal residual stenosis and a nonflow limiting type A dissection. Coronary angiography was performed immediately after balloon angioplasty (B) and at 2 months due to atypical chest discomfort (C) and at the 9-month follow-up (D). After balloon angioplasty, OCT revealed that the lumen was relatively well expanded, although the disrupted plaque was created (E). At 2 months, OCT demonstrated an enlarged lumen; the dissected flap was no longer visible (F). At 9 months, the lumen was well preserved and the area of disrupted plaque was completely healed (G). OCT, optical coherence tomography; PCB, paclitaxel-coated balloon. *Small septal branch. Bar = 1 mm.
This prospective observational study shows that a strategy of balloon dilation followed by PCB for the treatment of de novo coronary lesions restores and maintains coronary blood flow by means of a short-term mechanical effect and a sustained pharmacological effect. Mechanically, balloon angioplasty dilates the lumen with concomitant compression and dissection of the plaque. This leads to an absolute increase in minimal lumen area to a value that no longer generates ischemia. Plain balloon angioplasty was originally developed as a revascularization therapy that restores coronary flow by intentional plaque modification.9 However, elastic recoil and restenosis were major limitations.10 In contrast, PCB was developed to deliver a single dose of paclitaxel during the 1-minute PCB inflation time that was proven in a preclinical trial.5 As the main effects of PCB rely on the rapid transfer of the antiproliferative agent to the vessel wall, paclitaxel was adopted for use in drug-coated balloons with prolonged tissue retention rates.11 Paclitaxel exerts potent antiproliferative effects by binding to the subunit of tubulin, resulting in the arrest of microtubule function and thus promoting prolonged antiproliferation.12 As a result, paclitaxel can inhibit arterial smooth muscle cell proliferation and migration after being used locally.13 Several randomized clinical trials have shown better angiographic outcomes of PCB treatment not only in in-stent restenosis compared with plain balloon angioplasty14 or DES,15 but also in small vessel disease compared with DES.16 The main pathophysiology of restenosis after balloon angioplasty is arterial remodeling and neointimal hyperplasia.17 A recent study showed that successful PCB treatment of de novo coronary arteries after predilatation led to late lumen increase.4 They suggested that by local drug release to the vascular wall, positive effects to reduce neointimal hyperplasia and even to increase vascular lumen were possible. Recently, we showed that PCB treatment for de novo lesions increased the vessel and lumen areas and decreased plaque burden after 9 months by intravascular ultrasound (in press), suggesting that arterial constriction was prevented with paclitaxel.
In this study, lumen area enlarged after 9 months, suggesting that both intimal hyperplasia and arterial constriction were prevented with coated paclitaxel use. Suppressed plaque progression or vascular remodelling associated with local paclitaxel delivery is a possible mechanism. Experimental animal studies have demonstrated that paclitaxel causes apoptosis and necrosis of endothelial and smooth muscle cells.18 Data from OCT showed regression of intimal volume in in-stent restenosis lesions, which can be explained by cytotoxic mechanisms.19 In addition, it is possible that the healing process of the intimal dissections caused by the balloon angioplasty can seal with shrinkage of the intimal tissue, without additional recurrent proliferation due to the cytostatic activity of paclitaxel.20 As a result, paclitaxel can inhibit arterial smooth muscle cell proliferation and migration after being used locally, leading to coronary patency. The results of this study suggest that luminal enlargement was obtained mainly during the mid-term follow-up period.
In the post-POBA OCT acquisitions, all of the lesions treated with PCB showed extensive dissections of the intima. Not all dissections were treated with stents because of the good angiographic results and acceptable FFR values above 0.8. Two thirds of dissections were healed at follow-up, resulting in improvement in the luminal symmetry. After the sealing and decrease in the size of the dissections, FFR value showed no significant change between post-POBA and the 9-month follow-up.
LimitationsFirstly, selection bias may have occurred in individual cases. Additionally, patients with an ongoing acute coronary syndrome were not considered eligible for inclusion due to the complex nature of the study (ie, pre- and postprocedural FFR and OCT). Hence, only elective patients were included in the study. Secondly, although clinical and angiographic outcomes are promising, the nature of this registry that selectively applied PCB based on the FFR measured after POBA does not allow for comparison with a reference technique. Nonetheless, this registry study might strengthen the results of previous PCB studies. Thirdly, the number of patients included was relatively low. The serial changes in the OCT images including predilated lesion data were available only for 9 lesions. Fourthly, OCT acquisition was conducted after POBA (just before PCB application) and the size of inflated PCB was significantly larger than that of the inflated balloon. Therefore, PCB might have additionally modified the predilated lesion compared with the acquired OCT images. However, in this study, very sensitive techniques were used that allow for accurate assessment of the short- and mid-term mechanisms involved in restoring and maintaining coronary blood flow. In addition, this study did not target all lesions of coronary artery disease, and therefore, the results cannot be applied to patients beyond the inclusion criteria and study protocol.
CONCLUSIONSThe PCB restores coronary blood flow by means of plaque modification, causing an increment in minimal lumen area. At serial mid-term follow-up with FFR and OCT, coronary flow was sustained and luminal enlargement with healed vessel was observed in PCB-treated de novo coronary lesions. Further studies to generalize this data would be necessary.
CONFLICTS OF INTERESTNone declared.
- –
The PCB produces a better angiographic outcome than DES in small vessel disease. However, the functional and morphological changes induced by PCB over time have not been fully explored in de novo coronary lesions.
- –
In selected lesions for PCB, coronary blood flow is maintained by means of luminal enlargement, and dissections after balloon angioplasty decrease or seal at mid-term follow-up. Further investigation is necessary to confirm these findings.
The authors wish to thank the nurses and technicians of the Department of Cardiology in Ulsan University Hospital for their cooperation. Especially, Jeom-Nam No who has contributed to the successful implementation of the program and performance of the study.