Lithoplasty has been demonstrated to be safe and effective in the treatment of moderately or severely calcified lesions of the femoropopliteal arteries, including chronic occlusions.1 Results have shown a significant reduction in stenosis, with a low need for stent implantation or revascularization at follow-up. More recently, experiences with lithoplasty have been published, with good outcomes for the treatment of calcified coronary lesions.2
There is little experience with the use of lithoplasty on peripheral vessels to allow facilitated transfemoral access in transcatheter aortic valve implantation.3 Evidence on its efficacy in this context would likely increase the percentage of patients treated via the transfemoral route—an important point given that this has been demonstrated to have a lower mortality rate and better outcomes than other access routes.4
We present our initial experience with 4 patients—which to the best of our knowledge is the largest series described so far—with severe, highly-calcified lesions in both iliac vessels, who underwent lithoplasty with the Shockwave Lithoplasty system (Shockwave Medical Inc).
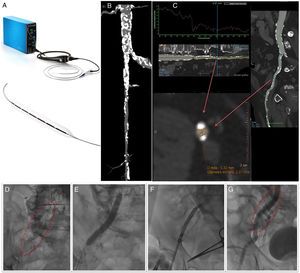
The Shockwave Lithoplasty balloon (Figure 1A) is a system that allows treatment of calcified stenotic lesions in peripheral arteries using the emission of sonic pressure waves that generate high mechanical energy. This energy cracks the superficial and deep calcium in the vessel with minimal impact on healthy tissue. The end objective is to convert the calcified plaque from a rigid, undilatable plaque to a more distensible plaque through which materials can be passed, minimizing trauma to the vessel compared with conventional balloon angioplasty.
A: Shockwave Lithoplasty system. B and C: computed tomography images showing extensive calcification of the common iliac artery and the origin of the right external iliac artery, as well as the common femoral artery, which also had severe lesions in all segments with a minimum lumen diameter of 5.3×2.4mm. D: extensive calcification observed on fluoroscopy. E: lithoplasty balloon inflated to 7×60mm, over a 330cm Asahi RG3 0.014-inch guidewire. F: advancing a CoreValve Evolute Pro 29mm prosthesis across the calcified lesion. G: final condition of the artery after finishing the procedure; the fracture produced in the calcium can be seen without images of associated dissection.
The device consists of a small control panel that contains the pulse generator and a small monitor where the pulses delivered are counted. The control panel is attached to a cable, which at the distal end has a button that activates and deactivates the delivery of pulses as required. This cable in turn connects to the lithoplasty balloon catheter. It is a semicompliant balloon, with an over-the-wire design, which contains 6 miniature sonic wave emitters. It is compatible with 6 or 7-Fr introducer sheaths (6.5 or 7mm balloons) and is mounted on a 0.014 inch guidewire. It is currently available in sizes from 3.5mm to 7mm, all of which have a balloon length of 60mm and total catheter length of 110mm. Each catheter can be used to deliver up to 10 cycles of 30 pulses (total 300 pulses per balloon). Once the desired position is achieved, to deliver the sonic waves, the balloon is first inflated to 4atm, 30 pulses are delivered, and then the pressure is increased to 6atm (nominal value); the burst pressure is 10atm. Since the energy propagation that leads to the calcium fragmentation is transmitted when the balloon comes into contact with the intima of the vessel, it is important to choose a balloon with a 1:1 ratio with the artery diameter to achieve good apposition between the balloon and the calcium of the lesion when the balloon is inflated to 4atm (2atm lower than the nominal value).
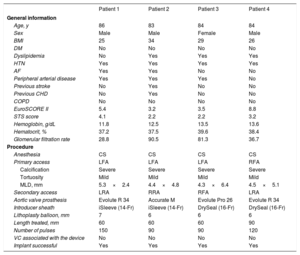
Table 1 shows the baseline characteristics of the patients and the procedure. Most patients were male with medium or high surgical risk, and previously undiagnosed peripheral arterial disease. All of the procedures were performed under conscious sedation, and in all bar one, radial access was chosen as secondary access, given the contralateral femoral disease. In most cases, the balloon length (60mm) covered the area to be treated and it was not necessary in any of the patients to use all of the 300 available pulses to achieve the desired result. There were no device-related complications.
Baseline characteristics of the patients and the procedure
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|
| General information | ||||
| Age, y | 86 | 83 | 84 | 84 |
| Sex | Male | Male | Female | Male |
| BMI | 25 | 34 | 29 | 26 |
| DM | No | No | No | No |
| Dyslipidemia | No | Yes | Yes | Yes |
| HTN | Yes | Yes | Yes | Yes |
| AF | Yes | Yes | No | No |
| Peripheral arterial disease | Yes | Yes | Yes | No |
| Previous stroke | No | Yes | No | No |
| Previous CHD | No | Yes | No | No |
| COPD | No | No | No | No |
| EuroSCORE II | 5.4 | 3.2 | 3.5 | 8.8 |
| STS score | 4.1 | 2.2 | 2.2 | 3.2 |
| Hemoglobin, g/dL | 11.8 | 12.5 | 13.5 | 13.6 |
| Hematocrit, % | 37.2 | 37.5 | 39.6 | 38.4 |
| Glomerular filtration rate | 28.8 | 90.5 | 81.3 | 36.7 |
| Procedure | ||||
| Anesthesia | CS | CS | CS | CS |
| Primary access | LFA | LFA | LFA | RFA |
| Calcification | Severe | Severe | Severe | Severe |
| Tortuosity | Mild | Mild | Mild | Mild |
| MLD, mm | 5.3×2.4 | 4.4×4.8 | 4.3×6.4 | 4.5×5.1 |
| Secondary access | LRA | RRA | RFA | LRA |
| Aortic valve prosthesis | Evolute R 34 | Accurate M | Evolute Pro 26 | Evolute R 34 |
| Introducer sheath | iSleeve (14-Fr) | iSleeve (14-Fr) | DrySeal (16-Fr) | DrySeal (16-Fr) |
| Lithoplasty balloon, mm | 7 | 6 | 6 | 6 |
| Length treated, mm | 60 | 60 | 60 | 90 |
| Number of pulses | 150 | 90 | 90 | 120 |
| VC associated with the device | No | No | No | No |
| Implant successful | Yes | Yes | Yes | Yes |
AF, atrial fibrillation; BMI, body mass index; CHD, coronary heart disease; COPD, chronic obstructive pulmonary disease; CS, conscious sedation; DM, diabetes mellitus; HTN, hypertension; LFA, left femoral artery; LRA, left radial artery; MLD, minimum lumen diameter; RFA, right femoral artery; RRA, right radial artery; STS: Society of Thoracic Surgeons; VC, vascular complication.
Figure 1 is taken from one patient. It shows computed tomography images of the iliofemoral vascular section prior to implantation and images from the procedure.
Based on the outcomes observed in our series, we can say that this is a simple, highly reproducible procedure that is effective in the treatment of severe, highly-calcified stenosis, as it allowed transfemoral implantation in all patients. Its usefulness in different types of vascular diseases remain to be determined.
Conflicts Of InterestR. Trillo Nouche is a proctor for Medtronic.