There is little data available for Spain on the outcomes of surgical treatment for severe tricuspid regurgitation. The aim of this study was to analyze clinical and echocardiographic outcomes in a series of patients who received surgical treatment for severe tricuspid regurgitation and to compare outcomes according to the operative approach to valve repair or replacement.
MethodsRetrospective study in 119 consecutive patients with severe tricuspid regurgitation undergoing valve surgery between April 1996 and February 2010.
ResultsA total of 61 ringless and 23 ring annuloplasties were performed and 11 bioprostheses and 24 mechanical prostheses were implanted. Perioperative mortality was 18.5% and was associated with age and cardiopulmonary bypass time. During clinical follow-up (median, 41 [interquartile range, 24-89] months), 2 reoperations were required in the ring annuloplasty and mechanical prosthesis groups; prosthetic thrombosis was diagnosed in 4 patients in the latter group. Total mortality after follow-up was 29.9% and was associated with age>70 years and extracorporeal circulation time. The emergence of new severe tricuspid regurgitation was associated with age and ringless annuloplasty (P=.04).
ConclusionsRingless repair was significantly associated with recurrence of severe tricuspid regurgitation. The use of mechanical prostheses was associated with a high rate of thrombosis. No significant differences in perioperative or total mortality were found between the different methods used for repair or valve replacement.
Keywords
Available data on the natural history of severe tricuspid regurgitation (TR) indicate a poor long-term prognosis. In most cases, etiology is functional and secondary to clinically predominant left valve disease,1 although TR may persist even after successful treatment of left valve disease.2 According to current clinical guidelines,3 there is still some debate as to the best time to initiate surgery and the most appropriate technique to use. Reconstructive surgery is generally recommended before valve replacement whenever technically feasible, as it leads to better outcomes.4 In Spain, there is little published data on the outcomes of surgery for severe TR or other valve pathologies, and available publications are all from the same study group.5–10 The aim of this study was to analyze clinical and echocardiographic outcomes in a series of patients who received surgical treatment for severe TR and to compare outcomes according to the methods used for valve repair or replacement.
METHODSStudy PopulationThis was a retrospective study that included 119 patients with severe TR who underwent surgery for tricuspid valve replacement or repair in our center between April 1996 and February 2010. A total of 1869 valve surgeries were performed over that period. The presence of a severe, symptomatic tricuspid lesion was taken as the indication for tricuspid valve surgery in all patients. The treatment strategy was decided by consensus between cardiologists, cardiac surgeons, and the patient. Repair was chosen whenever feasible, primarily because of the absence of significant organ disease. Type of repair was based on the surgeon's preference. From baseline to 2004, only ringless annuloplasties were used. From 2005 onwards, both types of repair were performed, in a 1:1 ratio. Between 1996 and 2004, only mechanical prostheses were implanted. From 2005 on, biological prostheses outnumbered mechanical prostheses by 2:1 and were the first choice in patients with no prior indication of permanent anticoagulation and whose limited life expectancy made the need for reoperation because of prosthetic degeneration unlikely. They were also the first choice in cases of reoperation for mechanical valve thrombosis.
Echocardiographic AssessmentEchocardiographic assessment was performed using either the Acuson Sequoia, the Acuson Aspen, or the Vingmed 750. Following American Society of Echocardiography recommendations,11 the standard exam used M-mode, two-dimensional pulsed, and continuous color Doppler modes, viewed in the usual planes. TR was defined as severe when a regurgitation jet extended over >30% of the area of the right atrium, with inadequate leaflet coaptation, and inverted systolic flow in the hepatic veins.12 Systolic pulmonary artery pressure was estimated from peak TR jet velocity.
Follow-upResults were analyzed in terms of postoperative morbidity and mortality, and residual TR. Clinical and echocardiographic follow-up was based on data obtained during routine visits to the cardiology outpatient department. Perioperative mortality was defined as occurring within 30 days of surgery or during hospitalization for surgery. Postoperative complications analyzed included infectious (endocarditis and mediastinitis), neurological (focal neurological deficit persisting for more than 24h and confirmed by computed tomography), pulmonary (intubation for more than 1 week, pneumonia, and pneumothorax), and renal (kidney failure, defined as elevated serum creatinine above 2mg/dL or 50% increase compared to baseline) complications, reoperation for bleeding, and low cardiac output syndrome. The occurrence of at least one of these postoperative complications was defined as a “surgical complication”. All-cause mortality, the need for new tricuspid surgery, prosthetic thrombosis, and development of new severe TR were assessed during follow-up.
Statistical AnalysisQualitative variables were expressed as percentages; time-dependent variables as medians [interquartile range], and other quantitative variables as means (standard deviation). We used the chi-square test to compare qualitative variables and Student's t test for continuous variables. Significance was set at P<.05. We developed a multiple logistic regression model to identify independent predictors of perioperative mortality and calculated the odds ratio (OR) and 95% confidence intervals (95%CI). The Cox regression method was used to analyze variables related to occurrence of severe TR during follow-up and to calculate hazard ratios (HR) and their corresponding 95%CI. We also used a Cox regression model to identify independent predictors of total mortality after follow-up and to estimate the corresponding HR and 95%CI. Survival analysis was performed using the Kaplan-Meier method.
RESULTSBaseline CharacteristicsRingless annuloplasty was performed in 61 patients using the De Vega technique and ring annuloplasty in 23; 11 patients received a bioprosthesis and 24 a mechanical prosthesis.
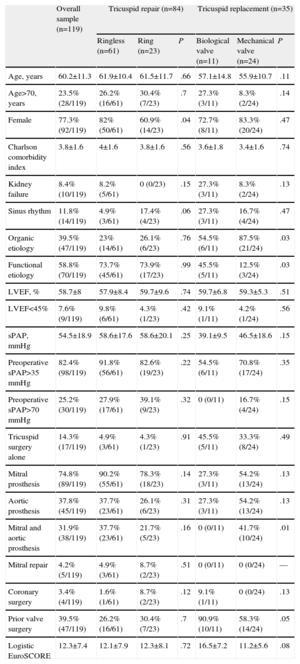
Table 1 shows the baseline characteristics of patients according to the type of repair or valve replacement. The etiology of the tricuspid condition was organic in 39.5% of cases, functional in 58.8%, and unknown in 1.7%. Of the cases with organic disease, 41 were of rheumatic origin, 2 had right-sided infective endocarditis, 2 were secondary to prolapse, 1 was congenital (Ebstein's malformation), and 1 was secondary to anorectics. Only 14.3% of the interventions exclusively involved the tricuspid valve and up to 31.9% involved implanting mitral and aortic prostheses; 39.5% of patients had undergone a previous valve repair or replacement. This prior valve surgery group included 64.7% of the patients who only had tricuspid surgery, compared to 35.3% of those who had multivalvular surgery (P=.02).
Baseline Characteristics
| Overall sample (n=119) | Tricuspid repair (n=84) | Tricuspid replacement (n=35) | |||||
| Ringless (n=61) | Ring (n=23) | P | Biological valve (n=11) | Mechanical valve (n=24) | P | ||
| Age, years | 60.2±11.3 | 61.9±10.4 | 61.5±11.7 | .66 | 57.1±14.8 | 55.9±10.7 | .11 |
| Age>70, years | 23.5% (28/119) | 26.2% (16/61) | 30.4% (7/23) | .7 | 27.3% (3/11) | 8.3% (2/24) | .14 |
| Female | 77.3% (92/119) | 82% (50/61) | 60.9% (14/23) | .04 | 72.7% (8/11) | 83.3% (20/24) | .47 |
| Charlson comorbidity index | 3.8±1.6 | 4±1.6 | 3.8±1.6 | .56 | 3.6±1.8 | 3.4±1.6 | .74 |
| Kidney failure | 8.4% (10/119) | 8.2% (5/61) | 0 (0/23) | .15 | 27.3% (3/11) | 8.3% (2/24) | .13 |
| Sinus rhythm | 11.8% (14/119) | 4.9% (3/61) | 17.4% (4/23) | .06 | 27.3% (3/11) | 16.7% (4/24) | .47 |
| Organic etiology | 39.5% (47/119) | 23% (14/61) | 26.1% (6/23) | .76 | 54.5% (6/11) | 87.5% (21/24) | .03 |
| Functional etiology | 58.8% (70/119) | 73.7% (45/61) | 73.9% (17/23) | .99 | 45.5% (5/11) | 12.5% (3/24) | .03 |
| LVEF, % | 58.7±8 | 57.9±8.4 | 59.7±9.6 | .74 | 59.7±6.8 | 59.3±5.3 | .51 |
| LVEF<45% | 7.6% (9/119) | 9.8% (6/61) | 4.3% (1/23) | .42 | 9.1% (1/11) | 4.2% (1/24) | .56 |
| sPAP, mmHg | 54.5±18.9 | 58.6±17.6 | 58.6±20.1 | .25 | 39.1±9.5 | 46.5±18.6 | .15 |
| Preoperative sPAP>35mmHg | 82.4% (98/119) | 91.8% (56/61) | 82.6% (19/23) | .22 | 54.5% (6/11) | 70.8% (17/24) | .35 |
| Preoperative sPAP>70mmHg | 25.2% (30/119) | 27.9% (17/61) | 39.1% (9/23) | .32 | 0 (0/11) | 16.7% (4/24) | .15 |
| Tricuspid surgery alone | 14.3% (17/119) | 4.9% (3/61) | 4.3% (1/23) | .91 | 45.5% (5/11) | 33.3% (8/24) | .49 |
| Mitral prosthesis | 74.8% (89/119) | 90.2% (55/61) | 78.3% (18/23) | .14 | 27.3% (3/11) | 54.2% (13/24) | .13 |
| Aortic prosthesis | 37.8% (45/119) | 37.7% (23/61) | 26.1% (6/23) | .31 | 27.3% (3/11) | 54.2% (13/24) | .13 |
| Mitral and aortic prosthesis | 31.9% (38/119) | 37.7% (23/61) | 21.7% (5/23) | .16 | 0 (0/11) | 41.7% (10/24) | .01 |
| Mitral repair | 4.2% (5/119) | 4.9% (3/61) | 8.7% (2/23) | .51 | 0 (0/11) | 0 (0/24) | — |
| Coronary surgery | 3.4% (4/119) | 1.6% (1/61) | 8.7% (2/23) | .12 | 9.1% (1/11) | 0 (0/24) | .13 |
| Prior valve surgery | 39.5% (47/119) | 26.2% (16/61) | 30.4% (7/23) | .7 | 90.9% (10/11) | 58.3% (14/24) | .05 |
| Logistic EuroSCORE | 12.3±7.4 | 12.1±7.9 | 12.3±8.1 | .72 | 16.5±7.2 | 11.2±5.6 | .08 |
LVEF, left ventricular ejection fraction; sPAP, systolic pulmonary artery pressure.
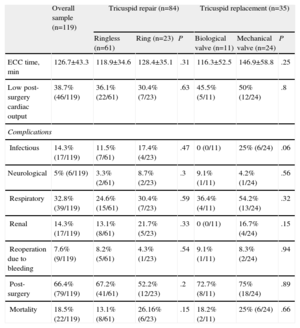
Table 2 summarizes cardiopulmonary bypass time, postoperative complications, and perioperative mortality by type of repair or valve replacement. No significant differences were observed for any of these parameters between the two types of repair or replacement. The perioperative mortality rate was 18.5% (22 patients).
Surgical Outcomes by Type of Valve Surgery or Replacement
| Overall sample (n=119) | Tricuspid repair (n=84) | Tricuspid replacement (n=35) | |||||
| Ringless (n=61) | Ring (n=23) | P | Biological valve (n=11) | Mechanical valve (n=24) | P | ||
| ECC time, min | 126.7±43.3 | 118.9±34.6 | 128.4±35.1 | .31 | 116.3±52.5 | 146.9±58.8 | .25 |
| Low post-surgery cardiac output | 38.7% (46/119) | 36.1% (22/61) | 30.4% (7/23) | .63 | 45.5% (5/11) | 50% (12/24) | .8 |
| Complications | |||||||
| Infectious | 14.3% (17/119) | 11.5% (7/61) | 17.4% (4/23) | .47 | 0 (0/11) | 25% (6/24) | .06 |
| Neurological | 5% (6/119) | 3.3% (2/61) | 8.7% (2/23) | .3 | 9.1% (1/11) | 4.2% (1/24) | .56 |
| Respiratory | 32.8% (39/119) | 24.6% (15/61) | 30.4% (7/23) | .59 | 36.4% (4/11) | 54.2% (13/24) | .32 |
| Renal | 14.3% (17/119) | 13.1% (8/61) | 21.7% (5/23) | .33 | 0 (0/11) | 16.7% (4/24) | .15 |
| Reoperation due to bleeding | 7.6% (9/119) | 8.2% (5/61) | 4.3% (1/23) | .54 | 9.1% (1/11) | 8.3% (2/24) | .94 |
| Post-surgery | 66.4% (79/119) | 67.2% (41/61) | 52.2% (12/23) | .2 | 72.7% (8/11) | 75% (18/24) | .89 |
| Mortality | 18.5% (22/119) | 13.1% (8/61) | 26.16% (6/23) | .15 | 18.2% (2/11) | 25% (6/24) | .66 |
ECC, extracorporeal circulation.
An echocardiographic assessment of residual TR after surgery was carried out in all discharged patients. TR was mild or absent in all cases of valve replacement, so we compared moderate and severe regurgitation between groups defined by the type of repair. There were 5 cases of moderate regurgitation in patients treated with ringless annuloplasty (5 of 53 survivors, 9.4%) compared with 1 case among those treated with ring annuloplasty (1 of 17 survivors, 5.8%); there were no significant differences between groups (P=.51). There was one case of severe TR in the ring annuloplasty group (1/17 [5.8%]), and no cases in the ringless repair group (P=.1).
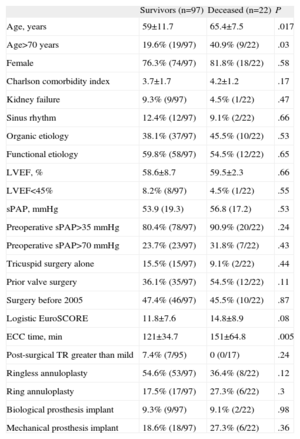
Univariate analysis (Table 3) showed that the only factors statistically associated with perioperative mortality were age (65.4 [7.5] vs 59 [11.7] years; P=.017), age>70 years, and cardiopulmonary bypass time (151 [64.8] vs 121 [34.7] min; P=.005). There were no significant differences in perioperative mortality by intervention type. Subsequent multivariate analysis included the logistic EuroSCORE as well as age and cardiopulmonary bypass time. Age (OR=1.08; 95%CI, 1.01-1.15; P=.02) and cardiopulmonary bypass time (OR=1.02; 95%CI, 1.01-1.03; P=.007) were significantly associated with perioperative mortality; logistic EuroSCORE was not associated (OR=1.02; 95%CI, 0.94-1.1; P=.66).
Univariate Analysis of Perioperative Mortality
| Survivors (n=97) | Deceased (n=22) | P | |
| Age, years | 59±11.7 | 65.4±7.5 | .017 |
| Age>70 years | 19.6% (19/97) | 40.9% (9/22) | .03 |
| Female | 76.3% (74/97) | 81.8% (18/22) | .58 |
| Charlson comorbidity index | 3.7±1.7 | 4.2±1.2 | .17 |
| Kidney failure | 9.3% (9/97) | 4.5% (1/22) | .47 |
| Sinus rhythm | 12.4% (12/97) | 9.1% (2/22) | .66 |
| Organic etiology | 38.1% (37/97) | 45.5% (10/22) | .53 |
| Functional etiology | 59.8% (58/97) | 54.5% (12/22) | .65 |
| LVEF, % | 58.6±8.7 | 59.5±2.3 | .66 |
| LVEF<45% | 8.2% (8/97) | 4.5% (1/22) | .55 |
| sPAP, mmHg | 53.9 (19.3) | 56.8 (17.2) | .53 |
| Preoperative sPAP>35 mmHg | 80.4% (78/97) | 90.9% (20/22) | .24 |
| Preoperative sPAP>70 mmHg | 23.7% (23/97) | 31.8% (7/22) | .43 |
| Tricuspid surgery alone | 15.5% (15/97) | 9.1% (2/22) | .44 |
| Prior valve surgery | 36.1% (35/97) | 54.5% (12/22) | .11 |
| Surgery before 2005 | 47.4% (46/97) | 45.5% (10/22) | .87 |
| Logistic EuroSCORE | 11.8±7.6 | 14.8±8.9 | .08 |
| ECC time, min | 121±34.7 | 151±64.8 | .005 |
| Post-surgical TR greater than mild | 7.4% (7/95) | 0 (0/17) | .24 |
| Ringless annuloplasty | 54.6% (53/97) | 36.4% (8/22) | .12 |
| Ring annuloplasty | 17.5% (17/97) | 27.3% (6/22) | .3 |
| Biological prosthesis implant | 9.3% (9/97) | 9.1% (2/22) | .98 |
| Mechanical prosthesis implant | 18.6% (18/97) | 27.3% (6/22) | .36 |
ECC, extracorporeal circulation; LVEF, left ventricular ejection fraction; sPAP, systolic pulmonary artery pressure; TR, tricuspid regurgitation.
Evolution of TR was assessed during follow-up echocardiography (median, 29 [interquartile range, 10.5-73.5] months) in 74 (76.3%) of the 97 survivors discharged home after surgery. Of these patients, 38 were treated with ringless annuloplasty (median follow-up, 39 [11-86] months) and 11 with ring annuloplasty (29 [10.5-73.5] months); 8 received biological prostheses (14 [8-32] months), and 17 received mechanical prostheses (52 [22.5-118.5] months). Thirteen patients in this group suffered serious TR; 11 had been treated with ringless annuloplasty (28.9% of patients followed up) and 2 with ring annuloplasty (18.2% of patients followed up). A total of 26.5% of survivors treated with valve repair suffered a new case of severe TR.
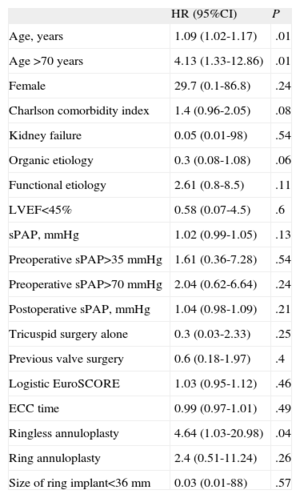
Table 4 shows the univariate analysis of severe TR during follow-up. Statistically significant associations were seen with age (P=.01), age>70 years (P=.01) and ringless annuloplasty (P=.04).
Univariate Analysis of Severe Tricuspid Regurgitation During Follow-up
| HR (95%CI) | P | |
| Age, years | 1.09 (1.02-1.17) | .01 |
| Age >70 years | 4.13 (1.33-12.86) | .01 |
| Female | 29.7 (0.1-86.8) | .24 |
| Charlson comorbidity index | 1.4 (0.96-2.05) | .08 |
| Kidney failure | 0.05 (0.01-98) | .54 |
| Organic etiology | 0.3 (0.08-1.08) | .06 |
| Functional etiology | 2.61 (0.8-8.5) | .11 |
| LVEF<45% | 0.58 (0.07-4.5) | .6 |
| sPAP, mmHg | 1.02 (0.99-1.05) | .13 |
| Preoperative sPAP>35mmHg | 1.61 (0.36-7.28) | .54 |
| Preoperative sPAP>70mmHg | 2.04 (0.62-6.64) | .24 |
| Postoperative sPAP, mmHg | 1.04 (0.98-1.09) | .21 |
| Tricuspid surgery alone | 0.3 (0.03-2.33) | .25 |
| Previous valve surgery | 0.6 (0.18-1.97) | .4 |
| Logistic EuroSCORE | 1.03 (0.95-1.12) | .46 |
| ECC time | 0.99 (0.97-1.01) | .49 |
| Ringless annuloplasty | 4.64 (1.03-20.98) | .04 |
| Ring annuloplasty | 2.4 (0.51-11.24) | .26 |
| Size of ring implant<36mm | 0.03 (0.01-88) | .57 |
95%CI, 95% confidence interval; ECC, extracorporeal circulation; HR, hazard ratio; LVEF, left ventricular ejection fraction; sPAP, systolic pulmonary artery pressure.
Clinical follow-up was conducted in 97.9% (n=95) of those who survived beyond the perioperative period. Median length of follow-up was 41 [24-89] months; 69.7% of survivors completed at least one year of follow-up and 58% completed at least 2 years. Those who completed long-term follow-up included 51 patients treated with ringless annuloplasty (median, 45 [21-103] months), 17 treated with ring annuloplasty (31 [8-77] months), 9 who received a biological prosthesis (17 [7-29] months), and 18 who received a mechanical prosthesis (52 [22.5 to 118.5] months).
Only 4 patients (4.2%) required reoperation of the tricuspid; 2 of those had been treated initially with ringless annuloplasty (3.9%) and 2 had received a mechanical prosthesis (11.1%). In both patients who received ringless annuloplasty, re-intervention was required because of the development of severe, symptomatic TR; in those who received a mechanical prosthesis it was due to thrombosis of the prosthesis.
Prosthetic thrombosis was observed in 4 patients (22.2% of those receiving mechanical prostheses); 2 of those patients had a single event, which was treated by fibrinolysis with good echocardiographic and clinical results (resolution) and no embolic complications. The other two patients suffered 2 and 3 events, respectively. In both cases, after the last thrombotic event, the mechanical prosthesis was replaced with a biological prosthesis.
Mortality during follow-up was 13.7% among patients who were alive at discharge and were not lost to follow-up (13 of 95 patients). Six of these patients had been treated with ringless annuloplasty (11.7%) and 1 with ring annuloplasty (5.9%); 1 had received a biological prosthesis (11.1%) and 5 a mechanical prosthetic (27.8%).
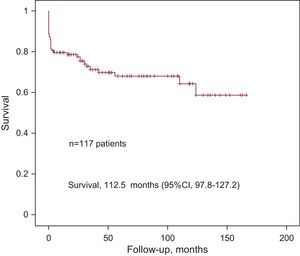
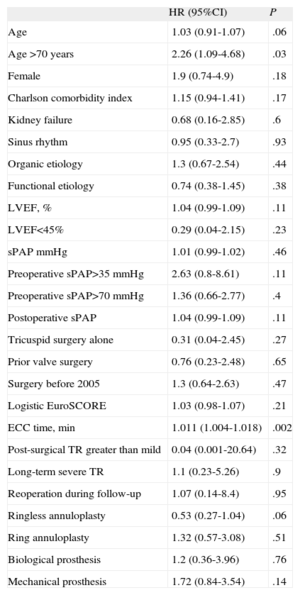
Total mortality (perioperative and follow-up) was 29.9% in patients not lost to follow-up (35 patients). The Figure shows the Kaplan-Meier survival curve. Univariate analysis (Table 5) showed that age >70 years (HR=2.26; 95%CI, 1.09-4.68; P=.03) and cardiopulmonary bypass time (HR=1.011; 95%CI, 1.004-1.018; P=.002) were significantly associated with total mortality. Both variables were included in a multivariate analysis that showed significant association of total mortality with age>70 years (HR=2.33; 95%CI, 1.06-5.11; P=.03) and cardiopulmonary bypass time (HR=1.01; 95%CI, 1.01-1.02; P=.002). No significant differences were found in a direct comparison of event-free survival between the two types of repair (ringless annuloplasty, 124.6 [95%CI, 105.9-143.3] months; ring annuloplasty, 35.9 [95%CI, 26.2-45.2] months; P=.2) and the two types of replacement (biological, 40.9 [95%CI, 25-56.8] months; mechanical, 86.5 [95%CI, 58.7-114.2]; P=.69).
Univariate Analysis of Total Mortality
| HR (95%CI) | P | |
| Age | 1.03 (0.91-1.07) | .06 |
| Age >70 years | 2.26 (1.09-4.68) | .03 |
| Female | 1.9 (0.74-4.9) | .18 |
| Charlson comorbidity index | 1.15 (0.94-1.41) | .17 |
| Kidney failure | 0.68 (0.16-2.85) | .6 |
| Sinus rhythm | 0.95 (0.33-2.7) | .93 |
| Organic etiology | 1.3 (0.67-2.54) | .44 |
| Functional etiology | 0.74 (0.38-1.45) | .38 |
| LVEF, % | 1.04 (0.99-1.09) | .11 |
| LVEF<45% | 0.29 (0.04-2.15) | .23 |
| sPAP mmHg | 1.01 (0.99-1.02) | .46 |
| Preoperative sPAP>35mmHg | 2.63 (0.8-8.61) | .11 |
| Preoperative sPAP>70mmHg | 1.36 (0.66-2.77) | .4 |
| Postoperative sPAP | 1.04 (0.99-1.09) | .11 |
| Tricuspid surgery alone | 0.31 (0.04-2.45) | .27 |
| Prior valve surgery | 0.76 (0.23-2.48) | .65 |
| Surgery before 2005 | 1.3 (0.64-2.63) | .47 |
| Logistic EuroSCORE | 1.03 (0.98-1.07) | .21 |
| ECC time, min | 1.011 (1.004-1.018) | .002 |
| Post-surgical TR greater than mild | 0.04 (0.001-20.64) | .32 |
| Long-term severe TR | 1.1 (0.23-5.26) | .9 |
| Reoperation during follow-up | 1.07 (0.14-8.4) | .95 |
| Ringless annuloplasty | 0.53 (0.27-1.04) | .06 |
| Ring annuloplasty | 1.32 (0.57-3.08) | .51 |
| Biological prosthesis | 1.2 (0.36-3.96) | .76 |
| Mechanical prosthesis | 1.72 (0.84-3.54) | .14 |
95%CI, 95% confidence interval; ECC, extracorporeal circulation; HR, hazard ratio; LVEF, left ventricular ejection fraction; sPAP, systolic pulmonary artery pressure; TR, tricuspid regurgitation.
Overall perioperative mortality in the present study was 18.5%, which is consistent with results described for tricuspid valve surgery, although published figures show substantial variability, with rates ranging from 0.713% to 50%.14 Nevertheless, perioperative mortality in the type of interventions studied here is generally lower than that seen in left valvular heart disease and cardiac surgery in general.15 The series that included what is likely the largest sample size in tricuspid disease had a perioperative mortality rate of 10.6%.16 The higher mortality rates in our study were conditioned by high levels of surgical risk, determined by several factors: mean logistic EuroSCORE of 12.3 (7.4), a small proportion of exclusively tricuspid interventions (14.3%), a high percentage of patients requiring mitral and aortic prostheses (31.9%), and a high proportion of second valve surgeries (39.5%). In the latter case, severe TR develops late in the natural history of left valve disease, despite surgical correction, either because it was not initially severe or not identified as organic. The poor outcomes associated with late tricuspid valve surgery have been widely reported.14,17,18 In this sense, the finding that tricuspid valve surgery alone was not associated with lower perioperative mortality could be due to the majority of these patients having previously undergone valvular surgery. Similarly, the association between prior valve surgery and tricuspid valve repair alone could explain why reoperation was not associated with greater perioperative mortality. The logistic EuroSCORE was close to a statistically significant association with perioperative mortality but did not quite achieve significance, probably due to an insufficient sample size.
The perioperative mortality rate in the present study does not improve upon the 7.6% reported in what we believe to be the largest Spanish series addressing the outcomes of surgery on the tricuspid valve, published by Bernal et al.6; which included 328 patients who had surgery between 1974 and 2005. However, the population in that study was only partially comparable to ours, given that stenosis was the predominant lesion in up to 28% of patients and etiology was rheumatic in all cases. Regarding the baseline characteristics of the series of Bernal et al., mean age at baseline was also lower by almost 9 years, 12.7% fewer patients had previously undergone cardiac surgery, 26.3% fewer patients received mitral prosthesis, and 12.2% fewer patients required an aortic prosthesis. The percentages of mitral and aortic repair were higher. The lower level of comorbidities was reflected in a mean extracorporeal circulation time 19.3min lower than in our study. These data suggest a possible change in the epidemiology of tricuspid valve disease in this context, with patients receiving surgery at a more advanced age and with more advanced valve disease, which is often associated with severe multiple valve disease and prior valve surgery. All of these factors could contribute to poorer surgical outcomes.
Multivariate analysis showed that perioperative mortality was only associated with age and cardiopulmonary bypass time. As reported, performing surgery when the patient is in functional class New York Heart Association III or lower can help optimize mortality rates.19
Clinical and Echocardiographic Follow-upFollow-up revealed severe TR in 26.5% of patients treated with valve repair. This is consistent with results obtained in other series.17,20 The association between the appearance of severe TR and ringless annuloplasty shown in univariate analysis leads us, like other authors,20–22 to conclude that ringless annuloplasty leads to poorer outcomes in follow-up than ring annuloplasty. It has been proposed that this may be due to the prosthetic ring protecting against dilation of the tricuspid valve annulus.21 The association between ringless annuloplasty and the severity of TR also affected the need for reoperation. Reoperation after earlier tricuspid valve repair has been associated with poor prognosis in the postoperative period and over the long term.5
When we compared mortality between ringless and ring annuloplasty, we observed a trend toward higher perioperative mortality in the latter but a trend towards higher mortality during follow-up in the former, although both differences were nonsignificant. The longer follow-up in the ringless annuloplasty group could play a role in the trend toward increased mortality. Few studies have compared long-term survival by type of repair, and most of them have been retrospective. The results have been contradictory, with some favoring ring annuloplasty21 and others indicating no significant differences between the two types of repair.23,24
Echocardiographic results after surgery for both types of valve replacement were excellent. During follow-up, there was not a single case of dehiscence in the bioprosthesis group. However, there was a high incidence (22.2%) of prosthetic thrombosis in those who received a mechanical prosthesis. This led to a need to reoperate in 11.1% of the mechanical prosthesis group and no such need in the bioprosthesis group. These results could be due in part to the uneven follow-up, with a longer follow-up in the mechanical prosthesis group. This could also explain why our rates of thrombosis and prosthetic dehiscence differ from those reported in the meta-analysis by Rizzoli et al.25 In that paper, rates of thrombosis and prosthetic dehiscence were similar, which led to similar rates of reoperation between the biological and mechanical prostheses groups.
When taking into account both perioperative mortality and mortality after follow-up, outcomes in the bioprosthesis group were superior to those in the mechanical prosthesis group, although the differences were not statistically significant. With regard to long-term survival after tricuspid valve replacement, the literature again provides conflicting data, with some studies indicating better outcomes for mechanical prostheses,19,26 while most have not found significant differences, as described in the two meta-analysis that have addressed the issue.25,27 Factors associated with good long-term outcome after tricuspid valve surgery include preoperative New York Heart Association class below IV7,19 and the absence of echocardiographic signs of early right heart failure such as decreased tricuspid annulus fractional shortening28 or pseudonormalization of the RIMP (right index of myocardial performance).19 It has also been suggested that the size of the tricuspid annulus, rather than just the presence or degree of TR, could be used as the indication for tricuspid valve surgery concomitant to left ventricular corrective surgery, a proposal which would lead to improvements in the natural history of this valve disease.13,28
LimitationsThis study suffers from the limitations inherent in any retrospective observational study. The relatively small sample size and the heterogeneous etiology of tricuspid valve disease and concomitant multi-valve disease in the study population make it difficult to generalize the results. The long-term echocardiographic follow-up was not completed in 23.7% of patients. The length of follow-up differed between the two repair and replacement groups, which limited direct comparisons between them. We had no data on right ventricular function, which may have influenced the results.
CONCLUSIONSSurgical treatment of severe TR in the context studied was associated with high perioperative mortality, which may have been at least partly due to patients being elderly, with multi-valve disease and prior valve surgery. Treatment with ringless annuloplasty was associated with the emergence of new severe TR during follow-up. Compared to biological prosthetic implants, mechanical prostheses led to increased morbidity in follow-up due to a high incidence of prosthetic thrombosis. No significant differences in total mortality by type of valve repair or replacement were observed.
CONFLICTS OF INTERESTNone declared.