The impact of atrial fibrillation on the prognosis of myocardial infarction is still the subject of debate. We analyzed the influence of previous and new-onset atrial fibrillation on in-hospital and long-term prognosis in patients with acute myocardial infarction.
MethodsProspective study of 4284 patients with ST-segment elevation acute myocardial infarction. We studied all-cause in-hospital and long-term mortality (median, 7.2 years) using adjusted models.
ResultsIn total, 3.2% of patients had previous atrial fibrillation and 9.8% had new-onset atrial fibrillation. In general, both groups of patients had a high baseline risk profile and an increased likelihood of in-hospital complications. The crude in-hospital mortality rate was higher in patients with previous atrial fibrillation than in those with new-onset atrial fibrillation (22% vs 12%; P<.001; 30% vs 10%; P<.001). The long-term mortality rate was 11.11/100 patient-years in patients with previous atrial fibrillation and 5.35/100 patient years in those with new-onset atrial fibrillation (both groups, P<.001). New-onset fibrillation alone (odds ratio=1.55; 95% confidence interval, 1.08-2.22) was an independent predictor of in-hospital mortality. Previous atrial fibrillation (hazard ratio=1.24; 95% confidence interval, 0.94-1.64) and new-onset atrial fibrillation (hazard ratio=0.98; 95% confidence interval, 0.80-1.21) were not independent predictors of long-term mortality.
ConclusionsNew-onset atrial fibrillation during hospitalization is an independent risk factor for in-hospital mortality in acute myocardial infarction.
Keywords
Atrial fibrillation (AF) is probably the most common arrhythmia in the general population.1 It is often undertreated2 and is an unexceptional finding (2%-22%) in acute myocardial infarction.3,4 The impact of AF on in-hospital prognosis and after discharge has been the subject of a protracted debate over the last decade. Some studies have shown that AF is independently associated with mortality,5–19 other studies have not found an association,20 and some studies have found an association between AF and better prognosis.21 It has also been found that AF during hospitalization (new-onset AF) can have an adverse impact on prognosis in contrast to preexisting AF (previous AF).8
The conceptual problem underlying this debate is based on viewing AF as a simple marker of heart failure (HF) or as a causal agent that could worsen coronary circulation and ventricular function and contribute to increased neurohumoral activation.22 Atrial fibrillation may also increase the incidence of severe ventricular tachyarrhythmias.23
In recent years, optimization of the treatment of ST-segment elevation myocardial infarction (STEMI) and especially the increase in reperfusion therapies have improved prognosis by decreasing HF and mortality, and thus a decrease in AF would be expected.24 A study of time trends in the onset of HF and AF during hospitalization may help to clarify their relationship.
The aim of this study was to analyze the prognostic significance of in-hospital previous AF, in-hospital new-onset AF, and long-term postdischarge AF in unselected patients with STEMI admitted to hospital. A secondary aim was to analyze the temporal evolution of new-onset AF and its relation to the onset of HF during hospitalization.
METHODSRecruitmentWe performed an observational longitudinal prospective study of patients diagnosed with STEMI in the coronary care units of Hospital Universitario Virgen de la Arrixaca (Murcia, Region of Murcia, Spain) and Hospital Universitario de Santa Lucía (Cartagena, Region of Murcia, Spain). The patients were recruited between January 1998 and January 2008.
The STEMI was defined as typical chest pain of ≥ 30min duration and/or elevated myocardial necrosis markers with ST-segment elevation in ≥ 2 precordial leads > 0.2mm in V1, V2, or V3 and > 0.1mm in lateral leads (aVL, I) or inferior leads (II, III, and aVF) or presumed new-onset left bundle branch block. The study was approved by the ethics committee and patients gave their written consent.
Definitions of VariablesAtrial fibrillation was defined as any electrocardiographically documented irregular ventricular rhythm in a 12-lead recording without a clear definition between the p and f waves (flutter). Previous AF was defined as a previously documented diagnosis of AF (electrocardiogram or medical history) and new-onset AF was defined as AF that appeared during hospitalization without a prior diagnosis of AF. New-onset AF was subdivided according to the time of onset: within 24h of hospitalization (≤ 24h) or after more than 24h of hospitalization (> 24h).
Severe or major bleeding complications were defined as bleeding in the brain, retroperitoneum, or any other location leading to hemodynamic deterioration and/or the need for transfusion of whole blood or blood components.
Angioplasty during hospitalization was defined as the composite of primary angioplasty and angioplasty performed during hospitalization, ie, delayed angioplasty or angioplasty performed the day after successful fibrinolysis. Heart rupture was defined as the composite of free wall rupture, ventricular septal rupture, or ruptured mitral chordae tendineae.
All patients underwent long-term postdischarge follow-up (median, 7.2 years) by telephone contact, review of medical records, visits to outpatient clinics, and review of death records. In-hospital mortality was excluded for the purpose of this analysis. The follow-up rate was 98%.
Statistical AnalysisMultivariate binary logistic regression was performed to analyze the factors associated with in-hospital mortality and new-onset AF during hospitalization. Odds ratios (OR) and 95% confidence intervals (95%CI) were calculated. The chi-square test was used to assess the significance of each variable in the prediction model of new-onset AF. Survival analysis was performed using the Kaplan-Meier method and the Mantel-Haenszel test. Hierarchical Cox multivariate regression models were used to study postdischarge mortality and the hazard ratio (HR) and the 95%CI were estimated. Non-Gaussian variables were transformed to their base-decimal logarithm. The model was adjusted by using the enter method with all covariates entered as blocks, including those considered relevant in the literature: age, sex, body mass index, diabetes mellitus, hypertension, current smoking, dyslipidemia (block 1); chronic obstructive pulmonary disease, newly diagnosed tumors (< 1 year), chronic kidney failure, previous ischemic heart disease, previous stroke, previous New York Heart Association functional class ≥ II (block 2); heart rate and systolic blood pressure on arrival at the emergency department, Killip class, left ventricular ejection fraction on hospitalization (block 3); revascularization with angioplasty and fibrinolysis (block 4); and HF during hospitalization (> 24h) (block 5). The log-linear and proportional hazards assumptions were checked by graphical methods. Final model discrimination and calibration were determined using the C-statistic and the Hosmer-Lemeshow test, respectively. The Harrell C-statistic was calculated for the Cox models.
The linear trends test was used to analyze trends and 5 two-year periods were established according to the time of recruitment: period 1 (1998-1999), period 2 (2000-2001), period 3 (2002-2003), period 4 (2004-2005), and period 5 (2006-January 2008). Missing values per variable were generally < 2% for most variables (99%). A P value of < .05 was used as a cutoff for statistical significance. Analyses were conducted using PASW, version 20 (IBM, Unites States) and STATA 9.1 (College Station, Texas, United States).
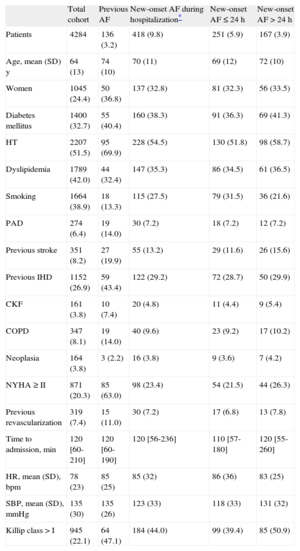
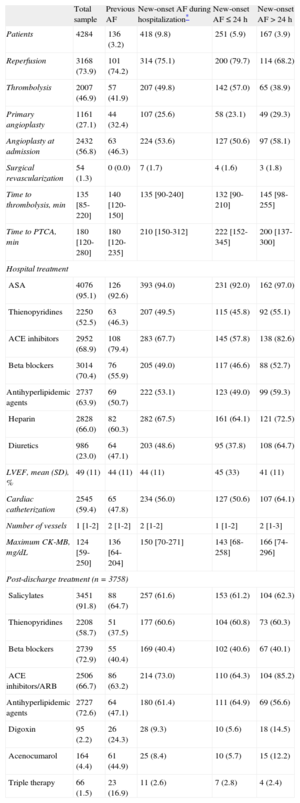
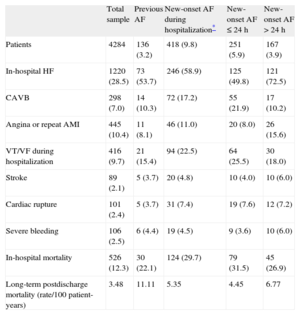
RESULTSBaseline Characteristics of the SampleTable 1 shows the baseline characteristics of the study sample (n=4284). The mean age was 64 years; 24.0% were women. In total, 3.2% of the patients had previous AF and 9.8% developed new-onset AF (60.0% in the first 24h). After 24h, new-onset AF occurred in 80.8% before the end of the third day. Postdischarge AF persisted in 8.7% of the patients with new-onset AF who survived hospitalization. Table 2 shows hospital treatment including reperfusion therapy and treatment at discharge. Table 3 shows in-hospital complications.
Baseline Characteristics. Patients’ antecedents and Clinical Status on Admission
| Total cohort | Previous AF | New-onset AF during hospitalization* | New-onset AF ≤ 24 h | New-onset AF>24 h | |
| Patients | 4284 | 136 (3.2) | 418 (9.8) | 251 (5.9) | 167 (3.9) |
| Age, mean (SD) y | 64 (13) | 74 (10) | 70 (11) | 69 (12) | 72 (10) |
| Women | 1045 (24.4) | 50 (36.8) | 137 (32.8) | 81 (32.3) | 56 (33.5) |
| Diabetes mellitus | 1400 (32.7) | 55 (40.4) | 160 (38.3) | 91 (36.3) | 69 (41.3) |
| HT | 2207 (51.5) | 95 (69.9) | 228 (54.5) | 130 (51.8) | 98 (58.7) |
| Dyslipidemia | 1789 (42.0) | 44 (32.4) | 147 (35.3) | 86 (34.5) | 61 (36.5) |
| Smoking | 1664 (38.9) | 18 (13.3) | 115 (27.5) | 79 (31.5) | 36 (21.6) |
| PAD | 274 (6.4) | 19 (14.0) | 30 (7.2) | 18 (7.2) | 12 (7.2) |
| Previous stroke | 351 (8.2) | 27 (19.9) | 55 (13.2) | 29 (11.6) | 26 (15.6) |
| Previous IHD | 1152 (26.9) | 59 (43.4) | 122 (29.2) | 72 (28.7) | 50 (29.9) |
| CKF | 161 (3.8) | 10 (7.4) | 20 (4.8) | 11 (4.4) | 9 (5.4) |
| COPD | 347 (8.1) | 19 (14.0) | 40 (9.6) | 23 (9.2) | 17 (10.2) |
| Neoplasia | 164 (3.8) | 3 (2.2) | 16 (3.8) | 9 (3.6) | 7 (4.2) |
| NYHA ≥ II | 871 (20.3) | 85 (63.0) | 98 (23.4) | 54 (21.5) | 44 (26.3) |
| Previous revascularization | 319 (7.4) | 15 (11.0) | 30 (7.2) | 17 (6.8) | 13 (7.8) |
| Time to admission, min | 120 [60-210] | 120 [60-190] | 120 [56-236] | 110 [57-180] | 120 [55-260] |
| HR, mean (SD), bpm | 78 (23) | 85 (25) | 85 (32) | 86 (36) | 83 (25) |
| SBP, mean (SD), mmHg | 135 (30) | 135 (26) | 123 (33) | 118 (33) | 131 (32) |
| Killip class > I | 945 (22.1) | 64 (47.1) | 184 (44.0) | 99 (39.4) | 85 (50.9) |
AF, atrial fibrillation; CKF, chronic kidney failure; COPD, chronic obstructive pulmonary disease; HR, heart rate; HT, hypertension; IHD, ischemic heart disease; NYHA, New York Heart Association; PAD, peripheral artery disease; SBP, systolic blood pressure; SD: standard deviation.
The variable “time to admission” was estimated as the time from the onset of the first chest symptom or leading symptom and hospital arrival.
Data are expressed as No. (%), median (standard deviation) or median [interquartile range].
Reperfusion, Hospital Treatment, and Postdischarge Treatment
| Total sample | Previous AF | New-onset AF during hospitalization* | New-onset AF ≤ 24 h | New-onset AF > 24 h | |
| Patients | 4284 | 136 (3.2) | 418 (9.8) | 251 (5.9) | 167 (3.9) |
| Reperfusion | 3168 (73.9) | 101 (74.2) | 314 (75.1) | 200 (79.7) | 114 (68.2) |
| Thrombolysis | 2007 (46.9) | 57 (41.9) | 207 (49.8) | 142 (57.0) | 65 (38.9) |
| Primary angioplasty | 1161 (27.1) | 44 (32.4) | 107 (25.6) | 58 (23.1) | 49 (29.3) |
| Angioplasty at admission | 2432 (56.8) | 63 (46.3) | 224 (53.6) | 127 (50.6) | 97 (58.1) |
| Surgical revascularization | 54 (1.3) | 0 (0.0) | 7 (1.7) | 4 (1.6) | 3 (1.8) |
| Time to thrombolysis, min | 135 [85-220] | 140 [120-150] | 135 [90-240] | 132 [90-210] | 145 [98-255] |
| Time to PTCA, min | 180 [120-280] | 180 [120-235] | 210 [150-312] | 222 [152-345] | 200 [137-300] |
| Hospital treatment | |||||
| ASA | 4076 (95.1) | 126 (92.6) | 393 (94.0) | 231 (92.0) | 162 (97.0) |
| Thienopyridines | 2250 (52.5) | 63 (46.3) | 207 (49.5) | 115 (45.8) | 92 (55.1) |
| ACE inhibitors | 2952 (68.9) | 108 (79.4) | 283 (67.7) | 145 (57.8) | 138 (82.6) |
| Beta blockers | 3014 (70.4) | 76 (55.9) | 205 (49.0) | 117 (46.6) | 88 (52.7) |
| Antihyperlipidemic agents | 2737 (63.9) | 69 (50.7) | 222 (53.1) | 123 (49.0) | 99 (59.3) |
| Heparin | 2828 (66.0) | 82 (60.3) | 282 (67.5) | 161 (64.1) | 121 (72.5) |
| Diuretics | 986 (23.0) | 64 (47.1) | 203 (48.6) | 95 (37.8) | 108 (64.7) |
| LVEF, mean (SD), % | 49 (11) | 44 (11) | 44 (11) | 45 (33) | 41 (11) |
| Cardiac catheterization | 2545 (59.4) | 65 (47.8) | 234 (56.0) | 127 (50.6) | 107 (64.1) |
| Number of vessels | 1 [1-2] | 2 [1-2] | 2 [1-2] | 1 [1-2] | 2 [1-3] |
| Maximum CK-MB, mg/dL | 124 [59-250] | 136 [64-204] | 150 [70-271] | 143 [68-258] | 166 [74-296] |
| Post-discharge treatment (n=3758) | |||||
| Salicylates | 3451 (91.8) | 88 (64.7) | 257 (61.6) | 153 (61.2) | 104 (62.3) |
| Thienopyridines | 2208 (58.7) | 51 (37.5) | 177 (60.6) | 104 (60.8) | 73 (60.3) |
| Beta blockers | 2739 (72.9) | 55 (40.4) | 169 (40.4) | 102 (40.6) | 67 (40.1) |
| ACE inhibitors/ARB | 2506 (66.7) | 86 (63.2) | 214 (73.0) | 110 (64.3) | 104 (85.2) |
| Antihyperlipidemic agents | 2727 (72.6) | 64 (47.1) | 180 (61.4) | 111 (64.9) | 69 (56.6) |
| Digoxin | 95 (2.2) | 26 (24.3) | 28 (9.3) | 10 (5.6) | 18 (14.5) |
| Acenocumarol | 164 (4.4) | 61 (44.9) | 25 (8.4) | 10 (5.7) | 15 (12.2) |
| Triple therapy | 66 (1.5) | 23 (16.9) | 11 (2.6) | 7 (2.8) | 4 (2.4) |
ACE, angiotensin-converting enzyme; AF, atrial fibrillation; ARB, angiotensin receptor blockers; ASA, acetylsalicylic acid; CK-MB, creatine kinase MB isoform; LVEF, left ventricular ejection fraction; PTCA, percutaneous transluminal coronary angioplasty; SD, standard deviation.
The percentage was calculated according to total fibrinolysis.
The variable “time to admission” was estimated as the time from the onset of the first chest symptom or leading symptom and hospital arrival. The variable “time to percutaneous transluminal coronary angioplasty” was defined as the time between the onset of the first chest symptom or leading symptom and the beginning of coronary angioplasty.
Data are expressed as No. (%), median (standard deviation) or median [interquartile range].
In-hospital Complications and Mortality and Long-term Mortality
| Total sample | Previous AF | New-onset AF during hospitalization* | New-onset AF ≤ 24 h | New-onset AF>24 h | |
| Patients | 4284 | 136 (3.2) | 418 (9.8) | 251 (5.9) | 167 (3.9) |
| In-hospital HF | 1220 (28.5) | 73 (53.7) | 246 (58.9) | 125 (49.8) | 121 (72.5) |
| CAVB | 298 (7.0) | 14 (10.3) | 72 (17.2) | 55 (21.9) | 17 (10.2) |
| Angina or repeat AMI | 445 (10.4) | 11 (8.1) | 46 (11.0) | 20 (8.0) | 26 (15.6) |
| VT/VF during hospitalization | 416 (9.7) | 21 (15.4) | 94 (22.5) | 64 (25.5) | 30 (18.0) |
| Stroke | 89 (2.1) | 5 (3.7) | 20 (4.8) | 10 (4.0) | 10 (6.0) |
| Cardiac rupture | 101 (2.4) | 5 (3.7) | 31 (7.4) | 19 (7.6) | 12 (7.2) |
| Severe bleeding | 106 (2.5) | 6 (4.4) | 19 (4.5) | 9 (3.6) | 10 (6.0) |
| In-hospital mortality | 526 (12.3) | 30 (22.1) | 124 (29.7) | 79 (31.5) | 45 (26.9) |
| Long-term postdischarge mortality (rate/100 patient-years) | 3.48 | 11.11 | 5.35 | 4.45 | 6.77 |
AF, atrial fibrillation; AMI, acute myocardial infarction; CAVB, complete atrioventricular block; HF, heart failure; VF, ventricular fibrillation; VT, ventricular tachycardia.
Unless otherwise indicated, data are expressed as No. (%).
Compared with patients without previous AF, those with previous AF were significantly older, mainly women, and more of them had diabetes and hypertension; however, there were fewer patients with dyslipidemia in this group and fewer were current smokers. Comorbidity was markedly higher in this group of patients, more of whom had a history of ischemic heart disease, stroke, peripheral artery disease, chronic kidney failure, chronic obstructive pulmonary disease, and New York Heart Association ≥ II (Table 1). Regarding hospital treatment, this group underwent fewer angioplasty procedures during hospitalization and were more often treated with angiotensin-converting enzyme inhibitors and diuretics. Patients in this group, however, were less often treated with beta blockers and statins. These patients also had worse left ventricular ejection fraction (Table 2).
At discharge, patients with previous AF were more often prescribed triple therapy, angiotensin-converting enzyme inhibitors, and digoxin but were less often prescribed salicylates, thienopyridines, and beta blockers (Table 2).
Baseline Characteristics of the Sample According to New-onset Atrial Fibrillation: PredictorsA total of 418 patients developed new-onset AF during hospitalization. Unadjusted predictors of new-onset AF were age, female sex, diabetes mellitus, a history of stroke, baseline New York Heart Association ≥ II, a higher heart rate at hospitalization and Killip class, higher levels of creatine kinase MB isoform, and a higher frequency of HF during hospitalization. Protective factors were dyslipidemia, current smoking, higher systolic blood pressure, and higher left ventricular ejection fraction (Table 1 of supplementary material). The only independent predictors in the model were age (per decimal logarithm in years, OR=266; 95%CI, 42-1673), systolic blood pressure (per decimal logarithm in mmHg, OR=0.04; 95%CI, 0.01-0.11), and HF during hospitalization (OR=2.49; 95%CI, 1.88-3.31). According to the chi-square statistic, the most significant predictor was HF during hospitalization, followed by age and systolic blood pressure (Table 1 of supplementary material).
In-hospital Complications and Mortality According to Previous Atrial Fibrillation or New-onset Atrial FibrillationPatients with previous AF had a higher rate of HF during hospitalization (54% vs 28%; P<.001) and a greater crude in-hospital mortality rate (22% vs 12%, P<.001) than patients without previous AF; however, no significant differences were found in other in-hospital complications. Patients with new-onset AF were more likely to develop HF during hospitalization (P<.001), complete atrioventricular block (P<.001), stroke (P<.001), heart rupture (P<.001), and in-hospital mortality (P<.001) (Table 3). The 2 most important causes of in-hospital mortality were cardiogenic shock and electromechanical dissociation in patients with previous AF (46.7% and 33.3%, respectively) and in patients with new-onset AF (63.7% and 19.4%, respectively).
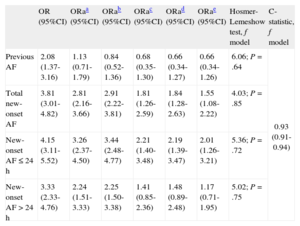
In a well-calibrated adjusted model (Table 4) with high discriminative power, new-onset AF during hospitalization (OR=1.55; 95%CI, 1.08-2.22) and new-onset AF within 24h (OR=2.01; 95%CI, 1.26-3.21) were independent predictors of in-hospital mortality. Previous AF (OR=0.55; 95%CI, 0.34-1.26) and new-onset AF > 24h (OR=1.17; 95%CI, 0.71-1.95) were not predictors of in-hospital mortality. The risk of death associated with new-onset AF and new-onset AF ≤ 24h was constant among patients who developed HF during hospitalization and those who did not (Table 2 of supplementary material) (interaction, P=.398 and P=.984).
Binary Logistic Regression Models for Hospital Mortality (Enter Method) With Incremental Adjustment for Confounders
| OR (95%CI) | ORaa (95%CI) | ORab (95%CI) | ORac (95%CI) | ORad (95%CI) | ORae (95%CI) | Hosmer-Lemeshow test, f model | C-statistic, f model | |
| Previous AF | 2.08 (1.37-3.16) | 1.13 (0.71-1.79) | 0.84 (0.52-1.36) | 0.68 (0.35-1.30) | 0.66 (0.34-1.27) | 0.66 (0.34-1.26) | 6.06; P=.64 | 0.93 (0.91-0.94) |
| Total new-onset AF | 3.81 (3.01-4.82) | 2.81 (2.16-3.66) | 2.91 (2.22-3.81) | 1.81 (1.26-2.59) | 1.84 (1.28-2.63) | 1.55 (1.08-2.22) | 4.03; P=.85 | |
| New-onset AF ≤ 24 h | 4.15 (3.11-5.52) | 3.26 (2.37-4.50) | 3.44 (2.48-4.77) | 2.21 (1.40-3.48) | 2.19 (1.39-3.47) | 2.01 (1.26-3.21) | 5.36; P=.72 | |
| New-onset AF>24 h | 3.33 (2.33-4.76) | 2.24 (1.51-3.33) | 2.25 (1.50-3.38) | 1.41 (0.85-2.36) | 1.48 (0.89-2.48) | 1.17 (0.71-1.95) | 5.02; P=.75 |
AF, atrial fibrillation; OR, odds ratio; ORa, adjusted odds ratio; 95%CI, 95% confidence interval.
Adjusted by age, sex, body mass index, and classic cardiovascular risk factors (family history of ischemic heart disease, diabetes mellitus, hypertension, current smoking, dyslipidemia).
Adjusted by the foregoing factors plus comorbidities (chronic kidney failure, chronic obstructive pulmonary disease, neoplasia, baseline New York Heart Association functional class ≥ II, ischemic heart disease, previous stroke, previous peripheral arterial disease).
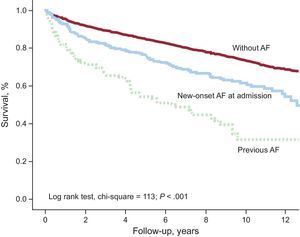
During follow-up (median, 7.2 years [interquartile range, 2.7-10.3]), the long-term mortality rate was 3.5/100 patient-years: 11.11/100 patient-years among patients with previous AF and 5.35/100 patient-years among patients with new-onset AF. Figure 1 shows the survival curve for patients with previous AF or new-onset AF.
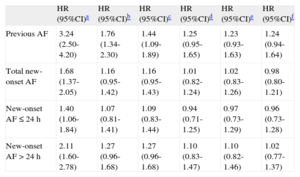
Table 5 shows that previous AF (HR=1.24; 95%CI, 0.94-1.64), total new-onset AF (HR=0.98; 95%CI, 0.80-1.21), new-onset AF ≤ 24h (HR=0.96; 95%CI, 0.73-1.28), and new-onset AF>24h (HR=1.02; 95%CI, 0.77-1.37) were not predictors of long-term mortality. The results presented did not change when stratified according to the development of HF during hospitalization (Table 3 of supplementary material) (interaction, P>.05 in all cases).
Cox Regression Models for Long-term Mortality (Enter Method) With Incremental Adjustment for Confounders
| HR (95%CI)a | HR (95%CI)b | HR (95%CI)c | HR (95%CI)d | HR (95%CI)e | HR (95%CI)f | |
| Previous AF | 3.24 (2.50-4.20) | 1.76 (1.34-2.30) | 1.44 (1.09-1.89) | 1.25 (0.95-1.65) | 1.23 (0.93-1.63) | 1.24 (0.94-1.64) |
| Total new-onset AF | 1.68 (1.37-2.05) | 1.16 (0.95-1.42) | 1.16 (0.95-1.43) | 1.01 (0.82-1.24) | 1.02 (0.83-1.26) | 0.98 (0.80-1.21) |
| New-onset AF ≤ 24 h | 1.40 (1.06-1.84) | 1.07 (0.81-1.41) | 1.09 (0.83-1.44) | 0.94 (0.71-1.25) | 0.97 (0.73-1.29) | 0.96 (0.73-1.28) |
| New-onset AF>24 h | 2.11 (1.60-2.78) | 1.27 (0.96-1.68) | 1.27 (0.96-1.68) | 1.10 (0.83-1.47) | 1.10 (0.82-1.46) | 1.02 (0.77-1.37) |
95%CI, 95% confidence interval; AF, atrial fibrillation; HR, hazard ratio.
Follow-up, median 7.2 [2.7-10.3] years.
Harrell c-statistic, f model=0.7933.
The proportional hazard assumption was checked before the hazard ratios were obtained for previous atrial fibrillation, total new-onset atrial fibrillation, new-onset atrial fibrillation, ≤ 24h, new-onset atrial fibrillation>24h using log-minus-log curves.
Adjusted by age, sex, body mass index, and classic cardiovascular risk factors (family history of ischemic heart disease, diabetes mellitus, hypertension, current smoking, dyslipidemia).
Adjusted by the foregoing factors plus comorbidities (chronic kidney failure, chronic obstructive pulmonary disease, neoplasia, baseline New York Heart Association functional class ≥ II, ischemic heart disease, previous stroke, previous peripheral arterial disease).
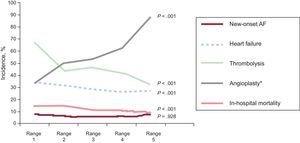
During the 10 years of recruitment (Figure 2), the rate of new-onset AF remained constant, whereas the rate of HF during hospitalization significantly decreased (period 1 vs period 5, 34% vs 27%; trend, P<.001). Angioplasty during hospitalization significantly increased (33.3% vs 88.2%; trend, P<.001). There was a substantial and significant decrease in in-hospital mortality (14.5% vs 9.6%; trend P<.001).
Trends in the rate of new-onset atrial fibrillation during hospitalization, heart failure during hospitalization, and reperfusion therapy. AF, atrial fibrillation. P values are presented for the trend test. AF, atrial fibrillation. *Composite of primary angioplasty and angioplasty performed during hospitalization (delayed angioplasty or angioplasty the day after successful fibrinolysis).
The clinical profile of patients with new-onset AF>24h differed from that of patients with new-onset AF<24h. The most significant differences (all P<.05) were older age, higher systolic blood pressure at hospitalization, worse Killip class, worse left ventricular ejection fraction, more diseased coronary vessels, reduced reperfusion, and an increased rate of in-hospital complications (complicated HF, complete atrioventricular block, and angina or reinfarction) (Tables 1–3). There was no significant difference between the groups in hospital mortality (P=.321), but the patients with new-onset AF>24h had higher long-term mortality (log rank test, chi square=4.60; P=.032).
DISCUSSIONThis study shows that in unselected patients admitted with a diagnosis of STEMI, new-onset AF is an independent risk factor for in-hospital mortality in contrast to previous AF. In the long-term, new-onset AF and previous AF were not independent factors for increased mortality. In addition, the rate of HF and in-hospital mortality significantly decreased during the study period, whereas the rate of new-onset AF remained constant.
Several studies have cited various predictors of new-onset AF. However, there is consensus that increased age and the rate of HF are the major risk factors,3,4 which is in line with the results of this study. In addition, other factors that may reflect certain hemodynamic changes associated with ventricular dysfunction, such as increased heart rate and some hypotension, have been shown to be predictors in this study and other studies.3,4
It has been suggested that AF increases the risk of morbidity and mortality3–19 and that this association is mediated to a greater or lesser extent by comorbidities (“the company it keeps”).3,4 Another key factor is the risk profile of the patient with acute coronary syndrome, as the lower the prognostic impact of AF, the higher the baseline risk.5 Furthermore, it has been suggested that new-onset AF, unlike previous AF, potentially entails higher in-hospital mortality, although new-onset AF may be an intermediate variable of HF.7,16,23 This study showed that in carefully adjusted models, new-onset AF— especially within the first 24 h–is predictive of in-hospital mortality. In addition, the association with an adverse prognosis was similar when stratified by HF. This finding is consistent with the GRACE (Global Registry of Acute Coronary Events)6 and others,7–10 but is not consistent with another study.20 In the OACIS study, Kinjo et al20 found no association between new-onset AF and in-hospital mortality (OR=1.42; 95%CI, 0.88-2.31). In our opinion, various differences between this study and our study may underlie the different findings. In contrast to the present study, Kinjo et al20 included patients with acute myocardial infarction with and without ST-segment elevation. Their study analyzed only patients undergoing cardiac catheterization, did not distinguish between patients with purely new-onset AF, ie, those without a previous history of AF, and finally, classified patients into the single category of atrial flutter and AF.
Regarding long-term postdischarge mortality, we did not find an independent association between previous AF and new-onset AF and all-cause mortality, which is in line with a previous study,19 but not with other studies (GUSTO–1,10 GUSTO–3,11 VALIANT (VALsartan In Acute myocardial iNfarcTion),9 OPTIMAAL,12 GISSI–3,13 and TRACE14). These studies found an independent effect of AF on postdischarge mortality, but were sub-analyses of studies conducted for other reasons that used specific or older patient populations with little comorbidity. They also used exclusion criteria and therefore differed from our study, which was an “all-comers” study. Some other differences are also relevant: a) in the OPTIMAAL,12 GUSTO-3,11 GISSI-3,13 and TRACE14 studies, AF and atrial flutter were grouped into the same category; b) the OPTIMAAL12 and GISSI-313 studies included any patients with acute myocardial infarction with or without ST-segment elevation and did not distinguish between patients who developed new-onset AF and those with previous AF, and c) adjustment for important confounders, such as comorbidity, in these studies was generally poor (GUSTO-3,11 and TRACE14) or nonexistent (GISSI-313). Of these studies, the model used in the VALIANT9 study had the best statistical adjustment, but that study did not distinguish between AF at admission and new-onset AF.
Our results contrast with those of the Cooperative Cardiovascular Project registry,15 which reported an independent prognostic impact of AF at hospitalization and after discharge. Unlike our study, this study included patients with acute myocardial infarction with and without ST-segment elevation, excluded all patients < 65 years, did not take patients with previous AF into account in the analysis, and only followed-up patients for 1 year.
In contrast to our study, Asanin et al16 found that new-onset AF>24h had an adverse effect on long-term mortality (7 years). However, this small study of 650 patients included infarctions with and without ST-segment elevation, and left ventricular ejection fraction and comorbidities were not considered in the multivariate model. Other registries also differ from our study because their adjustment for factors related to comorbidity was poor.17,21,25
To our knowledge, only 1 recent registry, the Worcester Heart Attack Study, has distinguished between previous AF and new-onset AF during hospitalization.18 In this study, the authors concluded that new-onset AF, especially permanent AF, may be associated with worse in-hospital and long-term prognosis. In contrast to our study, their study included patients with and without ST-segment elevation who underwent cardiac catheterization.
As mentioned, some studies have suggested that new-onset AF could simply be a reflection of the presence of HF3,22 and others have speculated that the rate of AF and its adverse prognostic impact could be reduced by optimized medical treatment.3 However, in line with another study,6 our time-trend analysis showed that the rate of new-onset AF remained almost constant over the 10-year recruitment period, despite a substantial and significant increase in coronary angioplasty and a reduction in HF. These results are consistent with those of a previous study, but differ from those of McManus et al,24 who reported that patients enrolled in the GRACE study showed a slight reduction in the rate of new-onset and previous AF parallel to a decrease in HF and in-hospital mortality. They attributed these findings to improvements in treatment. In contrast to our study, their study included patients with any type of acute coronary syndrome. A recent Spanish study that included patients with STEMI26 found a reduction in complicated AF, particularly in patients in the MASCARA registry27 compared with those in the PRIAMHO I and PRIAMHO II registries, as well as a reduction in HF. However, these patients differed from ours, mainly because there were fewer patients with diabetes and fewer comorbidities.
LimitationsNo information was available on the duration of AF during hospitalization or whether it was persistent or permanent, which may be of relevance.16
We cannot exclude the possibility that previous AF may be associated with long-term mortality and may therefore be clinically relevant, given the small number of patients with previous AF compared with the total sample, which gave rise to a type 2 error of 0.42 (HR=1.24).
CONCLUSIONSPrevious and new-onset AF are both markers of poor prognosis in STEMI, but only new-onset AF is an independent risk factor for in-hospital mortality both in patients who develop HF and those who do not.
CONFLICTS OF INTERESTNone declared.