We present the results of the Spanish Implantable Cardioverter-defibrillator Registry for 2015, as compiled by the Electrophysiology and Arrhythmia Section of the Spanish Society of Cardiology.
MethodsData collection sheets were voluntarily completed by each implantation team and prospectively sent to the Spanish Society of Cardiology.
ResultsThe number of reported implantations was 5465 (85% of the estimated total number of implantations). The implantation rate was 118 per million population while the estimated rate was 138. First implantations comprised 71.8%. Data were obtained from 169 hospitals (7 more than in 2014). Most implantations (82.7%) were performed in men. The mean patient age was 62.8±13.3 years. Most patients showed severe or moderate-to-severe ventricular dysfunction and were in New York Heart Association function class II. The most frequent cardiac condition was ischemic heart disease, followed by dilated cardiomyopathy. Implantations for primary prevention indications comprised 58.2%. Electrophysiologists performed 79.6% of the implantations.
ConclusionsThe 2015 Spanish Implantable Cardioverter-defibrillator Registry received information on 85% of the implantations performed in Spain. The number of implantations has grown from previous years. The percentage of implantations for primary prevention indications has slightly decreased from the previous registry.
Keywords
Implantable cardioverter-defibrillators (ICDs) are useful for the primary and secondary prevention of sudden cardiac death. The main indications for ICD implantation have been derived from numerous studies and have been included in the successive clinical management guidelines of patients with ventricular arrhythmias or at risk of sudden cardiac death.1–3 However, the increased use of ICD has raised questions about their effectiveness outside the setting of clinical trials, about the real-world selection of patients for implantation, and about the availability, safety, and cost-effectiveness of this therapy.4 Thus, given the scarcity of information in the medical literature on these aspects and the application of the clinical guidelines to unselected patient populations, health care registries could be extremely useful.
The current study presents the data on ICD implantations reported to the Spanish Implantable Cardioverter-defibrillator Registry in 2015. Most Spanish centers implanting ICDs have contributed to the registry. As in the previous official reports on this activity in Spain,5–14 this report has been prepared by the members of the Electrophysiology and Arrhythmia Section of the Spanish Society of Cardiology (Sociedad Española de Cardiología [SEC]).
The main aim of the registry is to determine the current implantation situation in Spain, with special emphasis on indications, patients’ clinical characteristics, implantation data, types of devices, programming, and procedural complications.
METHODSThe registry data were obtained using a data collection form available at the SEC website.15 Each implantation team directly and voluntarily completed this form during or after the procedure with the help of the technical staff of the ICD manufacturer.
A specially appointed technician introduced the information into the database of the Spanish Implantable Cardioverter-defibrillator Registry, with the help of a computer technician of the SEC and a member of the Electrophysiology and Arrhythmia Section. The computer technician and section member also performed data cleaning. The authors of this article analyzed the data and are responsible for this publication.
The census data for the distinct calculations of rates per million population, both national and by autonomous community and province, were obtained from the estimations of the Spanish National Institute of Statistics as of Friday, January 1, 2016.16
To estimate the representativeness of the registry, the proportion of implantations and replacements recorded in the registry was calculated with respect to the total number of implantations and replacements performed in Spain in 2015. This number was based on the data for 2015 reported to the European Confederation of Medical Suppliers Associations (Eucomed) by the suppliers of ICDs in Spain.17
If the data collection sheet recorded various clinical presentations or arrhythmias in the same patient, only the most serious condition was included in the analysis.
The percentages of each of the variables analyzed were calculated by taking into account the total number of implantations including information on the analysis variable.
Statistical AnalysisNumerical results are expressed as mean±standard deviation or median [interquartile range], according to the distribution of the variable. Continuous quantitative variables were compared using analysis of variance or the Kruskal-Wallis test. Qualitative variables were compared using the chi-square test. The relationships between the number of implantations and the devices implanted per million population and the total number of implantations and the number of implantations for primary prevention in each center were studied using linear regression models.
RESULTSThe response rates to the distinct fields of the data collection sheet ranged from 99.4% for the field “name of the implanting hospital” to 25.3% for the field “hospital of origin”.
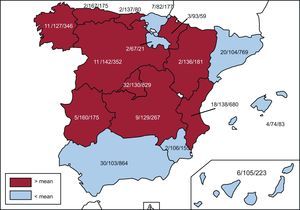
Implanting CentersA total of 169 hospitals performing ICD implantations reported their data to the registry (162 in 2014). The data from the 169 hospitals are shown in Table 1; 32 forms were excluded due to errors in the center records; 99 hospitals were public centers. The total number of implanting centers, rate per million population, and total number by autonomous community according to the data sent to the registry are shown in Figure 1. During 2015, only 14 centers implanted more than 100 devices; 85, 10 or fewer; and 33, only 1.
Implantations by Autonomous Community, Province, and Hospital
| Andalusia | ||
| Almería | Hospital Torrecárdenas | 25 |
| Hospital Vithas Virgen del Mar | 3 | |
| Cádiz | Hospital de Jerez | 37 |
| Hospital Ntra. Señora de la Salud | 2 | |
| Hospital San Carlos | 5 | |
| Hospital Universitario de Puerto Real | 9 | |
| Hospital Universitario Puerta del Mar | 36 | |
| Córdoba | Hospital de la Cruz Roja de Córdoba | 3 |
| Hospital Reina Sofía de Córdoba | 53 | |
| Granada | Hospital Clínico Universitario San Cecilio | 2 |
| Hospital Universitario Virgen de las Nieves | 89 | |
| Huelva | Hospital Blanca Paloma | 1 |
| Hospital Costa de la Luz | 3 | |
| Hospital General Juan Ramón Jiménez | 49 | |
| Jaén | Complejo Hospitalario de Jaén | 33 |
| Málaga | Clínica El Ángel | 9 |
| Clínica Parque San Antonio | 25 | |
| High Care San Antonio | 1 | |
| Hospital Internacional Xanit | 10 | |
| Clínica Benidorm | 1 | |
| Clínica Costa del Sol | 1 | |
| Clínica Quirón de Marbella | 9 | |
| Hospital Virgen de la Victoria | 221 | |
| Seville | Clínica Sagrado Corazón, S.A. | 3 |
| Hospital Viamed Santa Ángela de la Cruz | 2 | |
| Clínica Santa Isabel | 2 | |
| Hospital Nuestra Señora de Valme | 55 | |
| Clínica de Fátima | 1 | |
| Hospital Virgen del Rocío | 77 | |
| Hospital Virgen Macarena | 85 | |
| Aragon | ||
| Zaragoza | Hospital Clínico Universitario Lozano Blesa | 43 |
| Hospital Miguel Servet | 132 | |
| Hospital Quirón Zaragoza | 7 | |
| Principality of Asturias | Centro Médico de Asturias | 1 |
| Hospital Universitario Central de Asturias | 174 | |
| Balearic Islands | Clínica Rotger Sanitaria Balear, S.A. | 1 |
| Clínica USP Las Palmas | 7 | |
| Hospital Son Llàtzer | 18 | |
| Hospital Universitari Son Espases | 57 | |
| Canary Islands | ||
| Las Palmas | Hospital Dr. Negrín | 56 |
| Hospital Insular de Gran Canaria | 47 | |
| Sta. Cruz de Tenerife | Clínica Santa Cruz | 2 |
| Hospital Nuestra Señora de la Candelaria | 57 | |
| Hospital S. Juan de Dios Tenerife | 1 | |
| Hospital Universitario de Canarias | 60 | |
| Cantabria | Hospital Universitario Marqués de Valdecilla | 80 |
| Castile and León | ||
| Ávila | Hospital Nuestra Señora de Sonsoles | 22 |
| Burgos | Hospital Universitario de Burgos, S.A. (HUBU) | 53 |
| Leon | Clínica San Francisco | 1 |
| Hospital de León | 67 | |
| Hospital del Bierzo | 1 | |
| Salamanca | Complejo Hospitalario de Salamanca | 83 |
| Segovia | Hospital General de Segovia | 1 |
| Valladolid | Hospital Clínico Universitario de Valladolid | 93 |
| Hospital Sagrado Corazón de Jesús | 4 | |
| Hospital Río Hortega | 27 | |
| Castile-La-Mancha | ||
| Albacete | Clínica Recoletas de Albacete | 1 |
| Hospital General de Albacete | 50 | |
| Sanatorio Sta. Cristina | 1 | |
| Ciudad Real | Hospital General de Ciudad Real | 42 |
| IDC Salud Ciudad Real | 3 | |
| Cuenca | Hospital Virgen de la Luz | 4 |
| Guadalajara | Hospital General y Universitario de Guadalajara | 25 |
| Toledo | Hospital Nuestra Señora del Prado | 24 |
| Hospital Virgen de la Salud | 117 | |
| Catalonia | ||
| Barcelona | Capio Hospital General de Catalunya | 13 |
| Centre Cardiovascular Sant Jordi, S.A. | 1 | |
| Centro Médico Teknon | 1 | |
| Clínica Corachan | 1 | |
| Clínica Pilar Sant Jordi | 5 | |
| Clínica Quirón Barcelona | 1 | |
| Clínica Sagrada Família | 2 | |
| Fundació de G.S. de l’Hospital de la Santa Creu i Sant Pau | 130 | |
| Hospital Clínic de Barcelona | 218 | |
| Hospital de Bellvitge | 125 | |
| Hospital del Mar | 24 | |
| Hospital Germans Trias i Pujol | 68 | |
| Hospital Parc Taulí de Sabadell | 1 | |
| Hospital Sant Joan Despí Moisès Broggi | 2 | |
| Hospital Sant Joan de Déu | 7 | |
| Hospital Vall d’Hebron | 108 | |
| Girona | Hospital Josep Trueta | 1 |
| Lleida | Hospital Universitario Arnau de Vilanova | 25 |
| Tarragona | Hospital Universitario de Tarragona Joan XXIII | 29 |
| Hospital de Sant Pau i Santa Tecla | 2 | |
| Valencian Community | ||
| Alicante | Clínica Benidorm | 41 |
| Clínica Quirón de Torrevieja | 1 | |
| Clínica Vistahermosa | 4 | |
| Hospital General Universitario de Alicante | 179 | |
| Hospital General Universitario de Elche | 1 | |
| Hospital IMED Elche | 1 | |
| Hospital Mediterráneo | 2 | |
| Hospital Universitari Sant Joan d’Alacant | 46 | |
| Castellón | Hospital General de Castelló | 52 |
| Hospital Rey Don Jaime | 2 | |
| Valencia | Hospital Arnau de Vilanova | 1 |
| Hospital Clínico Universitario | 93 | |
| Hospital de Manises | 22 | |
| Hospital General Universitario | 71 | |
| Hospital Nisa 9 de Octubre | 1 | |
| Hospital Universitari de la Ribera | 38 | |
| Hospital Universitario Dr. Peset | 44 | |
| Hospital Universitario La Fe | 121 | |
| Extremadura | ||
| Badajoz | Clideba. IDC SALUD | 3 |
| Hospital de Mérida | 2 | |
| Hospital Infanta Cristina de Badajoz | 135 | |
| Cáceres | Complejo Hospitalario de Cáceres | 35 |
| Hospital San Pedro de Alcántara | 2 | |
| Galicia | ||
| A Coruña | Complejo Hospitalario Universitario A Coruña | 119 |
| Complejo Hospitalario Universitario de Santiago | 79 | |
| Hospital HM Modelo | 3 | |
| Ourense | Complejo Hospitalario de Ourense | 3 |
| Pontevedra | Complejo Hospitalario Universitario de Vigo (CHUVI) | 121 |
| Hospital Álvaro Cunqueiro | 10 | |
| Hospital Miguel Domínguez | 2 | |
| Hospital Povisa | 5 | |
| Hospital Provincial de Pontevedra | 1 | |
| La Rioja | Hospital de San Pedro | 20 |
| Hopital Viamed Los Manzanos | 1 | |
| Community of Madrid | Clínica La Luz | 5 |
| Clínica Ruber | 1 | |
| Fundación Hospital Alcorcón | 22 | |
| Fundación Jiménez Díaz. Clínica Nuestra Señora de la Concepción | 55 | |
| Grupo Hospital de Madrid | 14 | |
| Hospital 12 de Octubre | 98 | |
| Hospital Central de la Defensa | 33 | |
| Hospital Clínico San Carlos | 79 | |
| Hospital de Fuenlabrada | 10 | |
| Hospital del Henares | 1 | |
| Hospital de Torrejón | 21 | |
| Hospital de Villalba | 1 | |
| Hospital General Universitario Gregorio Marañón | 73 | |
| Hospital Infanta Elena de Valdemoro | 2 | |
| Hospital Infanta Leonor de Madrid | 33 | |
| Hospital La Zarzuela | 1 | |
| Hospital Los Madroños | 3 | |
| Hospital Nisa Prado de Aravaca | 2 | |
| Hospital Quirón San Camilo | 5 | |
| Hospital Quirón Madrid | 2 | |
| Hospital Ramón y Cajal | 96 | |
| Hospital Rey Juan Carlos | 18 | |
| Hospital San Rafael | 2 | |
| Hospital Severo Ochoa | 18 | |
| Hospital Sur de Alcorcón | 5 | |
| Hospital Universitario de Getafe | 8 | |
| Hospital Universitario La Paz | 85 | |
| Hospital Universitario Puerta de Hierro de Majadahonda | 117 | |
| Hospital Virgen de la Paloma | 11 | |
| Hospital Virgen del Mar | 2 | |
| Hospital VITHAS Nuestra Señora de América | 5 | |
| Sanatorio San Francisco de Asís | 2 | |
| Region of Murcia | Hospital General Universitario Morales Meseguer | 8 |
| Hospital General Universitario Reina Sofía | 18 | |
| Hospital General Universitario Santa Lucía | 15 | |
| Hospital Rafael Méndez | 20 | |
| Hospital Universitario Virgen de la Arrixaca | 90 | |
| Chartered Community of Navarre | Clínica Universitaria de Navarra | 37 |
| Clínica San Miguel IMQ | 1 | |
| Hospital Virgen del Camino | 1 | |
| Hospital de Navarra | 20 | |
| Basque County | ||
| Álava | Hospital Txagorrittxu | 7 |
| Hospital Universitario de Araba | 53 | |
| Guipúzcoa | Hospital Universitario Donostia | 20 |
| Vizcaya | Hospital de Basurto | 15 |
| Hospital de Cruces | 61 | |
| Hospital de Galdakao-Usansolo | 19 | |
| Hospital IMQ Zorrotzaurre | 2 | |
| No data | 32 | |
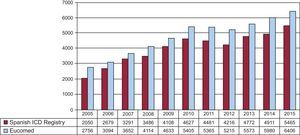
The total number of implantations (first implantations and replacements) in 2015 was 5465, more than in 2014 (total number, 4899). Because the Eucomed data17 showed a total number of devices of 6406, the registry figure represents 85% of the total. The total number of implantations reported to the registry and those estimated by Eucomed in the last 12 years are shown in Figure 2.
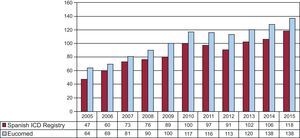
The overall rate of recorded implantations was 118 per million population; according to the Eucomed data, the rate was 138 per million population. The change in the implantation rate per million population during the last 12 years according to the registry and Eucomed data is shown in Figure 3. Implantations reported per implanting center are shown in Table 1.
The implanting hospital was recorded in 99.4% of cases. Most implantations (4958, 90.7%) were performed in public health care centers.
First Implantations vs ReplacementsThis information was available in 4853 forms sent to the SEC (88.8%). There were 3487 first implantations, representing 71.8% of the total (72.6% in 2014, 68.8% in 2013, 69.4% in 2012, 70.2% in 2011, and 73.8% in 2010). The rate of first implantations per million population was 75.1 in 2015 (79.0 in 2014, 63.8 in 2013, and 64.0 in 2012).
Age and SexThe mean age±standard deviation (range) of patients undergoing an ICD implantation or replacement was 62.8±13.3 (6-98) years in 2015, compared with 61.8±13.7 (7-94) years in 2014. The mean age of first implantation patients was 60.9±13.0 years. Most patients were men, who represented 82.7% of all patients and 83.3% of first implantation patients.
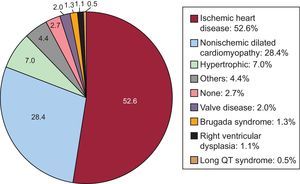
Underlying Heart Disease, Left Ventricular Ejection Fraction, Functional Class, and Baseline RhythmThe most frequent underlying cardiac condition in first implantation patients was ischemic heart disease (52.6%), followed by dilated cardiomyopathy (28.4%), hypertrophic cardiomyopathy (7.0%), valve diseases (2.0%), primary conduction abnormalities (Brugada syndrome and long QT syndrome) (1.8%), and arrhythmogenic dysplasias (1.1%) (Figure 4).
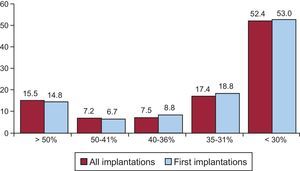
Left ventricular ejection fraction was > 50% in 15.5% of total patients, 50% to 41% in 7.2%, 40% to 36% in 7.5%, 35% to 31% in 17.4%, and < 30% in 52.4% (Figure 5). A similar distribution was seen in patients who underwent ICD replacement. These data were recorded in 72.4% of the data collection sheets of the registry.
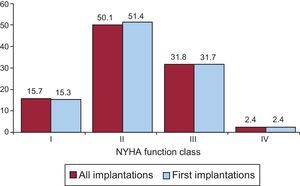
Most patients were in New York Heart Association (NYHA) functional class II (50.1%), followed by NYHA III (31.8%), NYHA I (15.7%), and NYHA IV (2.4%). For this parameter, the distribution was also similar between total implantations and first implantations (Figure 6), and these data were reported in 53.0% of the registry forms.
The baseline rhythm, reported in 43.7% of the patients, was largely sinusal (79.6%), followed by atrial fibrillation (15.6%) and pacemaker rhythm (4.5%); the remaining patients had other rhythms (atrial flutter and other arrhythmias).
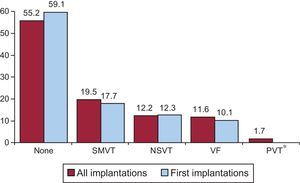
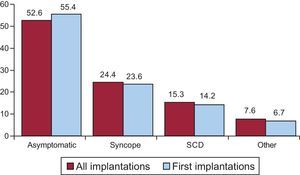
Clinical Arrhythmia Prompting Device Implantation, Its Form of Presentation, and the Arrhythmia Induced in the Electrophysiological StudyThese data were contained in 80.7% of the registry forms. For first implantations, most patients had no documented clinical arrhythmias (59.1%), followed by those with sustained monomorphic ventricular tachycardia, nonsustained ventricular tachycardia, and ventricular fibrillation (17.7%, 12.3%, and 10.1%, respectively). In total, patients with no documented clinical arrhythmia comprised 55.0% (Figure 7). The differences in the type of arrhythmia between the first implantation group and the total were not significant for any of the categories. The most frequent clinical presentation in both the total implantation group and the first implantation patients (67.3% and 71.7% of completed responses) was asymptomatic, followed by syncope, cardiac arrest, and “other symptoms” (Figure 8).
Data on electrophysiological studies were available for 2771 first implantation patients (79.5%). Such studies were performed in only 253 patients (9.18%). Sustained monomorphic ventricular tachycardia was the most common induced arrhythmia (49.5%), followed by ventricular fibrillation (14.8%) and, to a lesser extent, nonsustained ventricular tachycardia (15.5%) and others (3.0%). No arrhythmia was induced in 17.8% of the electrophysiological studies. Most of these studies were performed in patients with ischemic heart disease or dilated cardiomyopathy.
Clinical HistoryData on the clinical history of patients have only been available since 2011 because such data were not recorded in previous years.
Responses to questions on clinical history were obtained for between 67.0% and 83.9% of first implantation patients. The most important findings related to cardiovascular risk factors and history were as follows: hypertension, 59.1%; hypercholesterolemia, 48.0%; smoking, 35.6%; diabetes mellitus, 32.4%; history of atrial fibrillation, 27.4%; kidney failure, 13.2%; history of sudden cardiac death, 7.8%; and stroke, 5.9%.
The QRS width was recorded in 52.7% of the forms (mean, 127±46ms). In 32.3% of the patients, the recorded width was>140ms. Of these patients, 78.6% of the first implantation patients and 81.4% of the total had a defibrillator-resynchronization device (ICD-CRT [cardiac resynchronization therapy]).
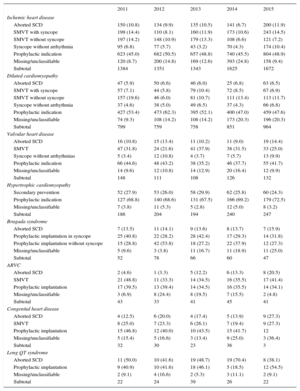
IndicationsThe changes in the type of heart disease and its presentation in first implantation patients from 2011 to 2015 are shown in Table 2. Data on this parameter were recorded in 91.1% of the registry sheets. For ischemic heart disease, the most frequent indication was primary prevention (48.9%), representing an increase from the previous year (45.5%) and a similar rate to 2013 (48.8%). For dilated cardiomyopathy, the main indication was also prophylactic (47.6% vs 47.0% in 2014, 52.1% in 2013, and 62.3% in 2012). For less common heart diseases, the most frequent indications were primary prevention of hypertrophic cardiomyopathy, valve diseases, congenital diseases, and Brugada syndrome. For long QT syndrome, prophylactic implantation was the most common indication (54.5%), in contrast to the data from 2014 (18.5%).
Number of First Implantations According to the Type of Heart Disease, Type of Clinical Arrhythmia, and Form of Presentation From 2011 to 2015
| 2011 | 2012 | 2013 | 2014 | 2015 | |
|---|---|---|---|---|---|
| Ischemic heart disease | |||||
| Aborted SCD | 150 (10.8) | 134 (9.9) | 135 (10.5) | 141 (6.7) | 200 (11.9) |
| SMVT with syncope | 199 (14.4) | 110 (8.1) | 160 (11.9) | 173 (10.6) | 243 (14.5) |
| SMVT without syncope | 197 (14.2) | 148 (10.9) | 179 (13.3) | 108 (6.6) | 121 (7.2) |
| Syncope without arrhythmia | 95 (6.8) | 77 (5.7) | 43 (3.2) | 70 (4.3) | 174 (10.4) |
| Prophylactic indication | 623 (45.0) | 682 (50.5) | 657 (48.8) | 740 (45.5) | 804 (48.9) |
| Missing/unclassifiable | 120 (8.7) | 200 (14.8) | 169 (12.6) | 393 (24.8) | 158 (9.4) |
| Subtotal | 1384 | 1351 | 1343 | 1625 | 1672 |
| Dilated cardiomyopathy | |||||
| Aborted SCD | 47 (5.9) | 50 (6.6) | 46 (6.0) | 25 (6.8) | 63 (6.5) |
| SMVT with syncope | 57 (7.1) | 44 (5.8) | 79 (10.4) | 72 (8.5) | 67 (6.9) |
| SMVT without syncope | 157 (19.6) | 46 (6.0) | 81 (10.7) | 111 (13.4) | 113 (11.7) |
| Syncope without arrhythmia | 37 (4.6) | 38 (5.0) | 49 (6.5) | 37 (4.3) | 66 (6.8) |
| Prophylactic indication | 427 (53.4) | 473 (62.3) | 395 (52.1) | 400 (47.0) | 459 (47.6) |
| Missing/unclassifiable | 74 (9.3) | 108 (14.2) | 108 (14.2) | 173 (20.3) | 196 (20.3) |
| Subtotal | 799 | 759 | 758 | 851 | 964 |
| Valvular heart disease | |||||
| Aborted SCD | 16 (10.8) | 15 (13.4) | 11 (10.2) | 11 (9.0) | 19 (14.4) |
| SMVT | 47 (31.8) | 24 (21.6) | 41 (37.9) | 38 (31.5) | 33 (25.0) |
| Syncope without arrhythmias | 5 (3.4) | 12 (10.8) | 4 (3.7) | 7 (5.7) | 13 (9.9) |
| Prophylactic indication | 66 (44.6) | 48 (43.2) | 38 (35.2) | 46 (37.7) | 55 (41.7) |
| Missing/unclassifiable | 14 (9.6) | 12 (10.8) | 14 (12.9) | 20 (16.4) | 12 (9.9) |
| Subtotal | 148 | 111 | 108 | 126 | 132 |
| Hypertrophic cardiomyopathy | |||||
| Secondary prevention | 52 (27.9) | 53 (26.0) | 58 (29.9) | 62 (25.8) | 60 (24.3) |
| Prophylactic indication | 127 (68.8) | 140 (68.6) | 131 (67.5) | 166 (69.2) | 179 (72.5) |
| Missing/unclassifiable | 7 (3.8) | 11 (5.3) | 5 (2.8) | 12 (5.0) | 8 (3.2) |
| Subtotal | 186 | 204 | 194 | 240 | 247 |
| Brugada syndrome | |||||
| Aborted SCD | 7 (13.5) | 11 (14.1) | 9 (13.6) | 8 (13.7) | 7 (15.9) |
| Prophylactic implantation in syncope | 25 (40.8) | 22 (28.2) | 28 (42.4) | 17 (29.3) | 14 (31.8) |
| Prophylactic implantation without syncope | 15 (28.8) | 42 (53.8) | 18 (27.2) | 22 (37.9) | 12 (27.3) |
| Missing/unclassifiable | 5 (9.6) | 3 (3.8) | 11 (16.7) | 11 (18.9) | 11 (25.0) |
| Subtotal | 52 | 78 | 66 | 60 | 47 |
| ARVC | |||||
| Aborted SCD | 2 (4.6) | 1 (3.3) | 5 (12.2) | 6 (13.3) | 8 (20.5) |
| SMVT | 21 (48.8) | 11 (33.3) | 14 (34.5) | 16 (35.5) | 17 (41.4) |
| Prophylactic implantation | 17 (39.5) | 13 (39.4) | 14 (34.5) | 16 (35.5) | 14 (34.1) |
| Missing/unclassifiable | 3 (6.9) | 8 (24.4) | 8 (19.5) | 7 (15.5) | 2 (4.8) |
| Subtotal | 43 | 33 | 41 | 45 | 41 |
| Congenital heart disease | |||||
| Aborted SCD | 4 (12.5) | 6 (20.0) | 4 (17.4) | 5 (13.9) | 9 (27.3) |
| SMVT | 8 (25.0) | 7 (23.3) | 6 (26.1) | 7 (19.4) | 9 (27.3) |
| Prophylactic implantation | 15 (46.8) | 12 (40.0) | 10 (43.5) | 15 (41.7) | 12 |
| Missing/unclassifiable | 5 (15.4) | 5 (16.6) | 3 (13.4) | 9 (25.0) | 3 (36.4) |
| Subtotal | 32 | 30 | 23 | 36 | 3 |
| Long QT syndrome | |||||
| Aborted SCD | 11 (50.0) | 10 (41.6) | 19 (48.7) | 19 (70.4) | 8 (38.1) |
| Prophylactic implantation | 9 (40.9) | 10 (41.6) | 18 (46.1) | 5 (18.5) | 12 (54.5) |
| Missing/unclassifiable | 2 (9.1) | 4 (16.6) | 2 (5.3) | 3 (11.1) | 2 (9.1) |
| Subtotal | 22 | 24 | 39 | 26 | 22 |
ARVC, arrhythmogenic right ventricular cardiomyopathy; SCD, sudden cardiac death; SMVT, sustained monomorphic ventricular tachycardia.
Data are expressed as No. (%).
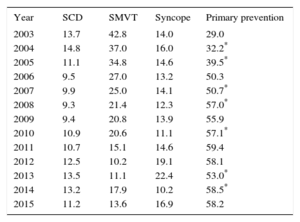
The implantation indication was reported in 65% of the records. Most first implantations were indicated for primary prevention (58.2%), a similar percentage to 2014. This variability has been growing and was statistically significant until 2008, between 2009 and 2010, and between 2013 and 2014 but was no longer significantly different between 2014 and 2015 (Table 3).
Changes in the Main Indications for Implantable Cardioverter-defibrillators (First Implantations, 2003-2015)
| Year | SCD | SMVT | Syncope | Primary prevention |
|---|---|---|---|---|
| 2003 | 13.7 | 42.8 | 14.0 | 29.0 |
| 2004 | 14.8 | 37.0 | 16.0 | 32.2* |
| 2005 | 11.1 | 34.8 | 14.6 | 39.5* |
| 2006 | 9.5 | 27.0 | 13.2 | 50.3 |
| 2007 | 9.9 | 25.0 | 14.1 | 50.7* |
| 2008 | 9.3 | 21.4 | 12.3 | 57.0* |
| 2009 | 9.4 | 20.8 | 13.9 | 55.9 |
| 2010 | 10.9 | 20.6 | 11.1 | 57.1* |
| 2011 | 10.7 | 15.1 | 14.6 | 59.4 |
| 2012 | 12.5 | 10.2 | 19.1 | 58.1 |
| 2013 | 13.5 | 11.1 | 22.4 | 53.0* |
| 2014 | 13.2 | 17.9 | 10.2 | 58.5* |
| 2015 | 11.2 | 13.6 | 16.9 | 58.2 |
SCD, sudden cardiac death; SMVT, sustained monomorphic ventricular tachycardia.
There was an 84.2% response rate to these questions. In 83.2%, the main implantation location was the electrophysiology laboratory (83.4% in 2014, 79.8% in 2013, 81.4% in 2012, and 76.4% in 2011), followed by the operating room (14.8%). Electrophysiologists performed 79.6% of implantations (81.7% in 2014, 80.7% in 2013, 81.0% in 2012, and 78.4% in 2011); surgeons, 9.6% (11.0% in 2014, 13.8% in 2013, 14.0% in 2012, and 15.5% in 2011); and both specialist types, 6.6%. Other specialists and intensivists were involved in 1.6% and 2.5%, respectively.
Generator Placement SiteInformation on the placement of first implantations was provided in 4221 registry forms (77.2%). Placement was subcutaneous in 97.6% of patients and subpectoral in the remaining 2.4%. The figures were 96.6% and 3.4% for all devices implanted, respectively.
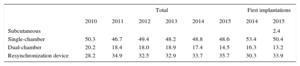
Device TypeThis information was available in 88.3% of the records and is summarized in Table 4. There were no differences in the type of device used compared with previous registries. For the first time, a significant number of subcutaneous defibrillator devices was implanted, 2.4% of first implantations.
Distribution Percentage of the Types of Devices Implanted
| Total | First implantations | |||||||
|---|---|---|---|---|---|---|---|---|
| 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2014 | 2015 | |
| Subcutaneous | 2.4 | |||||||
| Single-chamber | 50.3 | 46.7 | 49.4 | 48.2 | 48.8 | 48.6 | 53.4 | 50.4 |
| Dual-chamber | 20.2 | 18.4 | 18.0 | 18.9 | 17.4 | 14.5 | 16.3 | 13.2 |
| Resynchronization device | 28.2 | 34.9 | 32.5 | 32.9 | 33.7 | 35.7 | 30.3 | 33.9 |
In patients with ischemic heart disease, 71.5% of implants (76.8% in 2014, 74.8% in 2013, and 72.3% in 2012) were single- or double-chamber devices and 27.7% (23.1% in 2014, 25.5% in 2013, and 27.7% in 2012) were ICD-CRT devices. In patients with dilated cardiomyopathy, ICD-CRT devices comprised 55.6% (53.7% in 2014, 51.7% in 2013, and 56.5% in 2012).
Reasons for Device Replacement, Need for Lead Replacement, and Use of Additional LeadsOf the 1208 replacements, information was available on 1152 (95.3%). The most frequent reason for replacement was battery depletion (82.4%); complications motivated 8.6% (7.9% in 2013) and a change of indication prompted 9.5%. Of the 103 replacements due to a change of indication, 10.2% were required before 6 months (9.62% in 2014 and 11.6% in 2013).
Information was available on the status of the leads in 62.5% of the replacements; 8.1% were malfunctioning (68 records) and they were extracted in 35.4% of the patients reporting this problem.
Device ProgrammingInformation on this parameter was provided in 76.3% of records. The most widely used programming was VVI (52.4%), followed by DDD (30.3%), VVIR (6.5%), DDDR (5.6%), and other modes, largely algorithms to prevent ventricular pacing (5.1%).
Induction of ventricular fibrillation was tested in 122 patients, 2.7% of the 4504 records providing this information (2.9% in 2014, 5.1% in 2013, and 6.7% in 2012). The mean threshold was 23.6±8.9 J (19.7±6.8 in 2014, 20.4±6.5 in 2013, and 20.5±7.1 in 2012) and the mean number of shocks was 1.3.
ComplicationsWith a response rate of 76.2%, 29 complications were reported: 10 coronary sinus dissections, 3 pneumothoraces, 3 tamponades, 3 deaths, and 10 unspecified complications. The mortality rate was 0.07%; with 1 death more, this figure is slightly higher than that of the previous 2 years (0.05%).
DISCUSSIONThe 2015 data of the Spanish Implantable Cardioverter-defibrillator Registry continue to adequately reflect the implantation situation in Spain. The registry information is pertinent, particularly the number of implants, type of implant, indications, and patients’ clinical characteristics.
Comparison With Registries of Previous YearsThe Spanish Implantable Cardioverter-defibrillator Registry was first published in 2005 with the results of the 2002 to 2004 period.4 The number of implanted ICDs increased each year until 2010,5–10 with 2011 and 2012 then showing a lower total number of implantations, in both the registry11,12 and Eucomed data. In 201313 and 2014,14 the number of implantations rose again, exceeding the figures of the 2010 registry, and the number of implanted devices increased again in 2015. A continual increase was also seen in Europe, in both the number of ICDs and the number of ICD-CRTs.17
Whereas there was an increase in the percentage of implantations for primary indications in 2014,14 the current registry found a slight decrease (58.2% vs 58.5%). However, the percentage of implantations for primary indications has been largely unchanged since 2008 (Table 4).
There was an increase in the percentage of ICD-CRT implantations (35.7% vs 33.7% in 2014 and 32.9% in 2013). The percentage of single-chamber ICDs remained stable in 2015, showing few changes since 2011 (48.6% vs 48.8% in 2014 and 48.2% in 2013). The use of dual-chamber ICDs decreased (14.5% in 2015 vs 17.4% in 2014 and 18.9% in 2013), continuing the trend of recent years, which is probably due to the increased use of ICD-CRT. The resynchronization rate has slightly increased in recent years, and no major changes are expected, beyond a continuous slight year-on-year increase.
The most frequent indication in 2015 continued to be ischemic heart disease (52.4%), followed by dilated cardiomyopathy (28.4%). As in previous years,13,14 more than half of the devices implanted in patients with dilated cardiomyopathy were ICD-CRT devices (55.6%). The incidence was lower in patients with ischemic heart disease (27.7%) but higher than in 2014 (23.1%).
The progressive increase in the number of ICD implantations stopped in 2011 and 2012. The 2013 results showed a slight recovery, with the total number of implantations slightly surpassing the rate per million population of 2010 (102 vs 100).13 This increase was confirmed by the data for 2014 and 2015, with an implantation rate of 118 in our registry and of 138 according to the Eucomed data.17 In 2010, the implantation rate in Spain was about half of the European rate (116 vs 248); 5 years later, the gap has increased and the Spanish rate is now a third of the average European rate (138 vs 315).17
No recent studies have modified the ICD implantation indications. In 2002, the Multicenter Automatic Defibrillator Implantation Trial II18 study was published, followed by the Comparison of Medical Therapy, Pacing and Defibrillation in Heart Failure19 in 2005 and the Sudden Cardiac Death in Heart Failure Trial20 in 2006. These studies established the current indications in primary prevention and CRT and triggered a progressive increase in the number of implantations during that decade. The indications for ICD and CRT implantation are well supported in clinical practice guidelines.21–26 However, the implantation rate per million population does not correspond with that expected from the clinical evidence, both in Spain and in other European countries,27 a tendency that has become consolidated with time in these countries. As in previous registries, the 2015 registry represents 85% of the implantations reported to the Eucomed (82% in 2014). Most of the hospitals implanting ICDs provided the registry with data but 100% participation of the implanting centers remains to be reached. In addition, some data are lost while being sent and processed. All of these factors can explain the differences from the Eucomed data.
The number of implanting centers slightly increased from 2014. Two hospitals reported more than 200 implantations, 11 hospitals (11 in 2014 and 14 in 2013) reported more than 100 implantations, and 80 centers, mainly private, reported less than 10 implantations. The data show a tendency for an increase in implanting centers with low activity. Some studies have shown an inverse relationship between the implantation volume and the number of complications.28
There were no changes from previous registries in the epidemiological characteristics of the patients. Patients with severe ventricular dysfunction and in NYHA II and III continue to predominate. There were no differences from 2014 in the implantation setting—83.2% were performed in the electrophysiology laboratory (83.4% in 2014 and 79.8% in 2013)—or in the percentage of implantations performed by electrophysiologists (79.6% vs 81.7% in 2014 and 80.7% in 2013).
Differences Among Autonomous CommunitiesDifferences remain among autonomous communities. The implantation rate was 118 per million population according to the registry and 138 according to the Eucomed data; both databases showed an increase from 2014 (106 and 128, respectively). Several autonomous communities showed higher rates than the average: Principality of Asturias (167 implantations per million), Extremadura (160), Castile and León (142), Cantabria (137), the Valencian Community (136), Aragon (136), Community of Madrid (130), Castile-La-Mancha (129), and Galicia (127). The following were below the average: the Region of Murcia (106), the Canary Islands (105), Catalonia (104), Andalusia (103), Chartered Community of Navarre (93), the Basque Country (82), the Balearic Islands (74), and La Rioja (67). The difference between the communities with the highest and lowest rates of implantations is currently more than double (167 vs 67) and is higher than in the previous registry (153 vs 71). In general, all autonomous communities have increased their implantation rate and La Rioja has doubled its rate per million population vs 2014.
The 2015 data reaffirm the general increase in activity in Spain shown by the previous registry, confirming the tendency for a slow and progressive increase in the number of implantations per million. However, there was an increase in the differences among autonomous communities and compared with Europe. There was no association between the gross domestic product of the community and the number of implantations. Curiously, most high-income communities were below the mean. As in previous registries, the communities above the mean are the least populated, except for the Community of Madrid and the Valencian Community. There was also no relationship between the incidence of ischemic heart disease and heart failure in the various communities. There are other possible explanations for these differences, such as the health care organization of each community, the number of arrhythmia units, and the distribution of the referral hospitals.
Comparison With Other CountriesIncluding ICDs and ICD-CRTs, the implantation rate in the countries participating in the Eucomed was 315 per million population (302 in 2014). Germany, with 576 devices per million population, is still the country with the highest number of implantations. Spain (138 implantations per million) was the country with the lowest number of implantations. Several countries showed higher than average rates: the Netherlands (379 implantations per million), Italy (408), Denmark (281), and the Czech Republic (284). Below the average are Poland (306), Austria (288), Ireland (254), Belgium (235), Sweden (245), Norway (248), France (215), Switzerland (228), Finland (241), the United Kingdom (194), Portugal (189), Greece (158), and Spain (138). The difference in the implantation rate in Spain from the European mean decreased in 2015 (138 vs 315 and 126 vs 302 in 2014). The difference between Spain and the second-last country persists (138 vs 158).
The ICD implantation rate was 189 per million population in 2015 (183 in 2014). Germany (356 implantations per million population) had the highest number of implantations, whereas Spain (88) had the lowest.
The ICD-CRT implantation rate was 126 per million population (119 in 2014). Germany (220 implantations per million population) continued to be first, whereas Spain (50) had the lowest ICD-CRT implantation rate.
The proportion of ICD-CRT with respect to the total varies from 26% in Ireland, 29% in Poland, 46% in the Czech Republic, and 47% in the United Kingdom. The European average is 40%. Above the average are France, Switzerland, the United Kingdom, Italy, and the Czech Republic. Ireland and Poland are below 30%. Spain has a proportion of 36%.
These countries have the same regional differences29,30 seen in the Spanish registry, for unknown reasons. One possible explanation is the number of available arrhythmia units, but that does not explain the relationship, at least in Spain, because the communities with the highest number of available units had lower implantation rates. Other explanations, such as per capita income, also fail to show a correlation, with countries such as Ireland, the Czech Republic, and Poland showing much greater implantation rates than Spain. The prevalence of cardiovascular diseases, access to the health care system and its organization, and degree of acceptance of and adherence to the clinical practice guidelines could be related to the rate of implantations in Spain and its variability.
LimitationsThe registry included 85% of implantations performed in Spain according to the Eucomed data. This figure is higher than that of the previous year (82%). The percentage has decreased from 2007, when the representativeness was 90%. The number of registered implantations continues to accurately reflect the situation in Spain. The number of participating centers has remained practically unchanged in recent years.
The true number of implantations in some hospitals differs from that reported to the registry, given that the registry only includes received data collection sheets. Because data can now be sent in various ways, some sheets were not received or correctly registered. As the registry data were to be collected in 2 ways, on paper and via the Internet, 2015 was expected to be a year of transition. Unfortunately, despite being developed, the system allowing data to be collected via the Internet remains to be implemented. We hope that in 2016 all data will be collected via the website, which should improve the results and minimize the differences between the data obtained and those provided by the Eucomed.
There is excessive variability in the percentage of responses to the various questions in the ICD registry sheet, ranging from 99.4% for the implanting hospital to 52.7% for QRS width. Finally, the percentage of complications reported to the registry fails to reflect reality because these data are provided during or immediately after the implantation, meaning that most subacute complications are not recorded.
Future Prospects of the Spanish Implantable Cardioverter-defibrillator RegistryThis registry is the XII official report. The durability of this registry is a credit to all of the participating members of the Section of Electrophysiology and Arrhythmias of the SEC. The continued modernization of the registry will allow more and better information to be obtained with less effort on the part of the staff involved in its maintenance. The quality of the information will improve with further computerization of the registry, and the completion of certain fields will be obligatory. In the future, the system may permit more ambitious clinical objectives and include parameters such as death, shocks, and complications that will provide relevant clinical information.
CONCLUSIONSThe 2015 Spanish Implantable Cardioverter-defibrillator Registry collected information on 85% of all implantations performed in Spain and continues to be representative of the activity and current indications of this therapy in Spain. After 2 successive years of decreases in the number of implantations, the recovery seen in 2013 was confirmed in the last 2 years with a current figure of 118 per million population. As in previous years, the total number of implantations in Spain continues to be much lower than the average for the European Union, with the difference continuing to grow, and the autonomous communities continue to show considerable variability.
CONFLICTS OF INTERESTJ. Alzueta has participated in clinical studies funded by Medtronic, Boston, St. Jude, Biotronik, and Sorin and in roundtables sponsored by Boston. I. Fernández Lozano has participated in clinical studies funded by Medtronic, Boston, St. Jude, Biotronik, and Sorin and in roundtables sponsored by Boston and St. Jude. A. Barrera has participated in clinical studies funded by Medtronic, Boston, St. Jude, Biotronik, and Sorin.
We would like to thank all of the health care professionals involved in ICD implantation in Spain who have voluntarily and selflessly contributed to the ultimate success of the registry. We also thank José María Fernández, a SEC fellow who maintains the database of the Spanish Implantable Cardioverter-defibrillator Registry, for his enthusiastic work in maintaining the database and his participation in its development. Our thanks also go to the personnel at the ICD manufacturers (Medtronic, Boston Scientific, St. Jude Medical, Biotronik, and the Sorin Group) for their help in collecting and sending datasheets to the SEC for most of the implantations. Finally, we thank the SEC for its work in receiving the information, particularly Gonzalo Justes and José María Naranjo.