The NitOcclud Lê VSD Coil was specifically designed for transcatheter occlusion of ventricular septal defects (VSD) and became available for this purpose in August 2010. Our objective was to describe the Spanish experience of this technique and analyze its reliability and short- to mid-term efficacy.
MethodsNational multicenter observational study, which retrospectively recruited all patients (of any age) with VSD (of any location or type) who underwent percutaneous NitOcclud occlusion, using an intention-to-treat analysis, until January 2019.
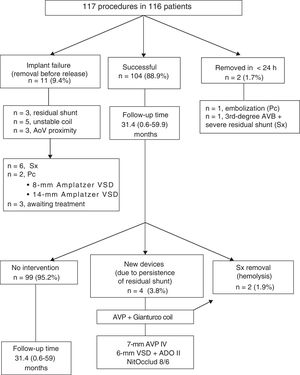
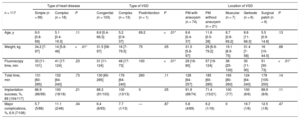
ResultsA total of 117 attempts were made to implant at least 1 NitOcclud in 116 patients in 13 institutions. The median [range] age and weight was 8.6 [0.4-69] years and 27 [5.8-97] kg, respectively. In 99 patients (85%), the VSD was an isolated congenital defect. The location was perimembranous in 95 (81%), and 74 (63%) of them were aneurysmatic. The mean fluoroscopy time was 34 [11.4-124] minutes. Of the 117 attempts, 104 were successful (89%) with a follow-up of 31.4 [0.6-59] months. At the last review, final complete occlusion of the defect without residual shunt or with only a minimal shunt was achieved in 92.3% (no shunt, n=73; trivial shunt, n=23). Four patients required a second procedure for residual shunt occlusion. Two devices had to be surgically explanted due to severe hemolysis. There were no deaths or other major complications.
ConclusionsThe NitOcclud device can be used successfully for a wide anatomical spectrum of VSD. The main issue is residual shunt, but its incidence decreases over time. The incidence of hemolysis was very low and no permanent changes were detected in atrioventricular conduction.
Keywords
Isolated ventricular septal defect (VSD), the most common congenital heart disease, accounts for up to 20% of heart defects at birth (3 of 1000 newborns).1 About 70% of VSDs are perimembranous in location and may variously extend to the conal or trabecular septum. Until the introduction of percutaneous occlusion techniques in 1988,2 any management required was exclusively surgical.2 The devices applied were initially designed for other uses (atrial septal defect or ductus closures) but Amplatzer (AGA Medical Corp, United States) developed specific and safer devices at the end of the 1990s, achieving outcomes comparable to those of surgery.3,4 However, the Amplatzer perimembranous VSD device was abandoned in many centers due to the unacceptable rate of atrioventricular block (AVB) (3.8%-22%).5–7 In later years, because surgical treatment also carries a potential risk of AVB,8,9 offlabel techniques were used for the percutaneous occlusion of VSDs with different devices, none of which were free from possible complications.
The NitOcclud Lê VSD Coil (PFM AG, Germany) was awarded the CE standard in August 2010. Compared with double-disc devices, the specific design of the coil represented a paradigm shift in occlusion technique (figure 1). A few series have reported its use10–15; most included a small number of patients and limited follow-up. The objective of this study was to describe the use of this technique in Spain and to analyze its short-to-mid-term reliability and efficacy.
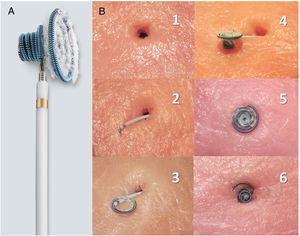
A: description of the device: nitinol coils in a diabolo structure with a central waist of 2.5mm in diameter and larger coils on the left than on the right; a polyester fiber covering on the left-hand side to improve thrombogenic capacity; available sizes: 8 × 6, 10 × 6, 12 × 6, 12 × 8, 14 × 8, and 16 × 8 mm, preassembled in a 6- to 7-Fr catheter with a transportation sheath for navigation. B: in vitro simulation of the closure technique (reproduced with permission of N.A. Haas); 1: defect; 2: transportation sheath traversing the defect, with the end of the left coil slightly exteriorized; 3: unfurling of the left-side coils; 4: unfurled left-side coils and waist (left side); 5: device positioned in the defect (left side); 6: device positioned in the defect (right side, right-side coils unfurled).
This retrospective, observational, national, multicenter study included all consecutive patients (of any age) with VSD (of any location or type) who underwent percutaneous occlusion with the NitOcclud Lê VSD Coil from the first use of the technique (September 2010) until January 2019.
Data were collected with a predefined questionnaire in accordance with Organic Law 3/2018 of December 5th, 2018, on the Protection of Personal Data and the Guarantee of Digital Rights. The study was approved by the research committee of the coordinating center and informed written consent was obtained from the patients or their legal representatives.
The criteria for patient selection were the presence of hemodynamically significant VSD, defined as that associated with dilatation of the left heart chambers (a sign of persistent volume overload) or with a Qp/Qs > 1.5. Other criteria were clinical symptoms of excessive pulmonary blood flow, pulmonary vascular resistance < 4 to 5 UW/m2, and the anatomical characteristics defined in figure 2 (a hemodynamic defect ≤ 8 mm). Ideally, patients selected for the treatment of perimembranous VSDs weighed > 10 kg. Many procedures were personally overseen by a proctor, in line with the training protocol recommended by the company. In general, the procedures were conducted under general anesthesia and with prophylactic cefazolin (2 mg/kg) (prior to and 8 and 16hours after the procedure), a bolus dose of 100 UI/kg heparin, and simultaneous echocardiographic monitoring (transthoracic or transesophageal) (figure 3). The femoral artery was used to access perimembranous and outlet VSDs, whereas the right jugular vein was used for apical and midmuscular defects. Qp/Qs measurement was not compulsory and was performed according to the standard practice of each hospital.
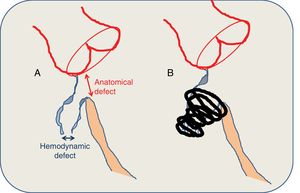
A: anatomical criteria of the defect. B: closure characteristics. The defect is measured via a combination of transesophageal echocardiography and left ventriculography. Perimembranous ventricular septal defects have a typically conical morphology, with the hemodynamic defect (which opens to the right ventricle) smaller than the anatomical defect (which can be seen from the left ventricle). The size of the device is calculated by considering that the left coil should have twice the diameter of the hemodynamic defect and be at least 1 to 2mm larger that the anatomical defect. Ideally, the coil should be situated in the interior of the aneurysm, away from the conducting tissue and the aortic cusps, avoiding clamping stress on the interventricular septum. In the absence of an aneurysmal sac, a superior margin of at least 3 to 4mm is required.
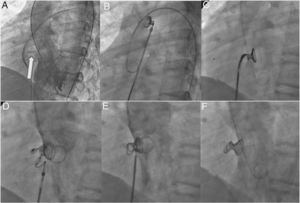
Phases of the implantation. A: left anterior oblique (55°) and cranial (30°) left ventriculography view for the visualization of a perimembranous ventricular septal defect with aneurysmal tissue (arrow). B: left coils and waist (1-2cm) unfurled in the ascending aorta in the catheter approaching from the right side of the heart, after performing an arteriovenous loop (with the lasso positioned in the trunk of the pulmonary artery and retrograde advance of the coronary catheter on the hydrophilic guidewire from the left side to the inferior vena cava, or superior if the access is jugular) and the advance of the sheath in close contact with the tip of the coronary catheter (kissing technique) to the distal aortic arch. C: the device in the left ventricle, positioned inside the defect after its descent through the aortic valvular area. D: unfurling of the right-hand coils. E: coil release. F: final angiogram with the coil located inside the aneurysmal tissue without interfering with the aortic valve or the residual shunt.
The procedure was considered successful if the device was positioned correctly and without major complications 24hours after the implantation.10,13 Major complications were those requiring a surgical or percutaneous intervention.
Patient follow-up was regularly performed in each center according to the specific characteristics of each patient, although it systematically included a physical examination, electrocardiography, and echocardiography. Other complementary tests, such as Holter ECG, were performed according to the results of clinical examinations. Data collection ended in July 2019. During the first 6 postimplantation months, 5 mg/kg/d aspirin was administered, as well as prophylactic antibiotics for infectious endocarditis.
Stata statistical software version 13.1 (United States) was used for data analysis. Continuous variables are presented as median [range] and categorical variables as frequencies (percentages). A t test was used to compare the mean age, weight, and times between 2 subgroups, whereas ANOVA was used for comparisons involving more than 2 subgroups. The Fisher exact test was used to compare success and complication percentages between subgroups. P values < .05 were considered significant.
RESULTSA total of 117 interventions were undertaken to implant at least 1 NitOcclud in 116 patients in 13 institutions (table 1). Outcomes are presented according to VSD subtype in table 2. The experiences of each hospital are shown in table 3. The viability of the procedure and its changes over time are presented in figure 4.
Patient, VSD anatomical, and general procedural characteristics
| Patient characteristics (n = 116) | VSD characteristics (n = 117) | Procedural characteristics (n = 117) | |||
|---|---|---|---|---|---|
| Age, yAge < 1 y | 8.6 (0.4-69.2)8 (6.8) | TypeCongenitalPostoperativePostinfarction | 103 (88.0)13 (11.1)1 (0.9) | TEE | 117 (100) |
| Femoral vein | 115 (98) | ||||
| Weight, kgWeight <10 kg | 27.5 [5.8-97]12 (8.6) | mPAP, mmHg | 17.5 [10-43] | ||
| Hospitalization ≤ 2 d | 89 (76) | ||||
| Size, cm | 127 (56-195) | LocationPM with aneurysmPMMuscularGerbodePostoperative residual | 74 (63.2)21 (18.0)7 (6.0)6 (5.1)9 (7.7) | Coil size8/610/612/612/814/816/8 | 34 (29.1)29 (24.8)18 (15.4)11 (9.4)14 (12.0)11 (9.4) |
| Type of heart diseaseIsolated VSDCCHD | 99 (85.3)17 (14.7) | ||||
| IndicationLV dilatationHF/volume overloadQp/Qs (n = 92)Previous IEMild AR | 113 (97.4)22 (19.0)1.57 (1-3)2 (1.7)15 (12.9) | ||||
| DefectsSingleMultiple | 78 (66.7%)39 (33.3%) | ||||
| Diameter, mmIn LVIn RV | 8.5 [3-20]4.5 [1.7-11] | Fluoroscopy time, min | 34 (11.4-124) | ||
| Procedural time, min | 133.5 [60-285] | ||||
AR, aortic regurgitation; CCHD, complex congenital heart disease; HF, heart failure; IE, infectious endocarditis; LV, left ventricle; mPAP, mean pulmonary arterial pressure; PM, perimembranous without tricuspid subvalvular apparatus-dependent aneurysmal tissue; RV, right ventricle; TEE, transesophageal echocardiography; VSD, ventricular septal defect.
Data are expressed as median [range] or No. (%).
Age and weight, procedural times and outcomes according to type of heart disease, and type and location of the VSD
| Type of heart disease | Type of VSD | Location of VSD | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n = 117 | Simple (n = 99) | Complex (n = 18) | P | Congenital (n = 103) | Complex (n = 13) | Postinfarction (n = 1) | P | PM with aneurysm (n = 74) | PM without aneurysm (n = 21) | Muscular (n = 7) | Gerbode (n = 6) | Surgical patch (n = 9) | P |
| Age, y | 9.0 [0.8-69] | 5.1 [0.4-19.3] | .11 | 8.6 [0.4-68.3] | 5.2 [0.9-37] | 69.2 | <.01* | 8.6 [0.4-37] | 11.6 [0.5-28] | 8.7 [0.8-69.2] | 8.6 [11-68.3] | 5.5 [0.9-14.8] | .13 |
| Weight, kg | 34.2 [7-97] | 14 [5.8-46] | <.01* | 31.5 [58-97] | 16 [7-79.5] | 75 | .05 | 31.5 [5.8-97] | 29 [6.9-79.2] | 19.1 [8.9-75] | 31.4 [7-58] | 16 [10-44.5] | .68 |
| Fluoroscopy time, min | 33 [11-101] | 41 [17-124] | .23 | 31 [11-124] | 48 [17-73] | 100 | <.01* | 29 [16-80] | 57 [19-124] | 38 [25-100] | 30 [11-90] | 51 [30-73] | <.01* |
| Total time, min | 131 [60-285] | 152 [64-240] | .73 | 130 [60-285] | 176 [64-240] | 260 | .11 | 128 [64-257] | 185 [60-285] | 165 [80-260] | 124 [64-240] | 178 [105-200] | .14 |
| Implantation success, %, 89 (104/117) | 86.9 (86/99) | 100 (18/18) | .21 | 88.3 (91/103) | 100 (13/13) | 0 | .05 | 91.9 (68/74) | 71.4 (15/21) | 100 (7/7) | 100 (6/6) | 88.9 (8/9) | .11 |
| Major complications, %, 6.9 (7/106) | 5.7 (5/88) | 11.1 (2/48) | .34 | 6.4 (6/93) | 7.7 (1/13) | — | .87 | 5.8 (4/69) | 6.2 (1/16) | 0 | 16.7 (1/6) | 12.5 (1/8) | .47 |
PM, perimembranous; VSD, ventricular septal defect.
Data are expressed as median [range] or % (n/N).
NitOcclud implantation experience according to hospital
| Hospital | A | B | C | D | E | F | G | H | I | J | K | L | M |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Program start | 29-5-2014 | 28-9-2010 | 10-4-2015 | 1-12-2015 | 14-7-2015 | 16-4-2015 | 22-6-2017 | 9-3-2018 | 11-2-2016 | 24-4-2016 | 22-3-2017 | 28-3-2017 | 4-4-2016 |
| Total (n = 117) | 42 | 37 | 9 | 8 | 5 | 4 | 3 | 2 | 2 | 2 | 1 | 1 | 1 |
| Age, y | 5.9 [0.4-15.5] | 5.5 [0.9-22.1] | 16.9 [2.3-37.1] | 13.6 [8.6-17.4] | 6.3 [1.6-13.3] | 5.7 [2.2-11.3] | 11.5 [8.7-14] | 9.8 14.9 | 6.9 11.6 | 10.3 18.1 | 11.8 | 69.2 | 68.3 |
| Weight, kg | 16.9 [5.8-79.2] | 22.6 [7-97] | 55 [8.9-79.5] | 39.2 [21-68] | 22.3 [10-68.5] | 16 [10-38] | 41.5 [32-45] | 54 84 | 21 32 | 38 55 | 51 | 75 | 58 |
| Coil size | |||||||||||||
| 8/6 | 19 | 10 | 1 | 3 | 1 | — | — | — | 1 | — | 1 | — | — |
| 10/6 | 6 | 15 | — | 2 | — | 2 | — | — | — | 1 | — | — | — |
| 12/6 | 7 | 5 | 1 | 1 | 2 | — | — | — | 1 | — | — | — | — |
| 12/8 | — | 4 | 3 | — | 2 | — | 1 | — | — | 1 | — | — | 1 |
| 14/8 | 3 | 3 | 3 | 2 | — | 2 | 1 | — | — | — | — | 1 | — |
| 16/8 | 7 | — | 1 | — | — | — | 1 | 2 | — | — | — | — | — |
| Nonimplanted patients (VSD location/type), 9.4% (11/117) | 7.1 (3/42)· Patient 8: Laubry· Patient 26: Laubry· Patient 29: Laubry | 13.5 (5/37)· Patient 4: PM with aneurysm. Patient 9: PM with aneurysm· Patient 13: PM with aneurysm· Patient 21: Laubry· Patient 35: PM | 0 | 12.5 (1/8)· Patient 4: PM | 0 | 25 (1/4)· Patient 3: PM with aneurysm | 0 | 0 | 0 | 0 | 0 | 1· Patient 1: Postinfarction muscular | 0 |
| Major complication, 6.9% (7/106) | 5.1 (2/39)· Patient 5: residual shunta· Patient 17: residual shunta | 9.4 (3/32)· Patient 1: hemolysisb· Patient 8: embolizationa· Patient 11: hemolysisa,b | 11 (1/9)· Patient 8: residual shunt and AVBb | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 (1/1)· Patient 1: hemolysisa |
| Successful, 89% (104/117) | 93 (39/42) | 84 (31/37) | 89 (8/9) | 87.5 (7/8) | 100 (5/5) | 75 (3/4) | 100 (3/3) | 100 (2/2) | 100 (2/2) | 100 (2/2) | 100 (1/1) | 0 | 100 (1/1) |
PM, perimembranous; VSD, ventricular septal defect.
Data are expressed as median [range] or % (n/N).
Despite the diversity of the clinical conditions, 89% of procedures were successful (104 of 117). Notably, the specific design of the device permitted the percutaneous occlusion of 6 Gerbode-type VSDs,16 9 residual VSDs over surgical patches in patients with complex congenital heart disease (CCHD), and 1 residual shunt in a muscular VSD previously closed with an Amplatzer device.
Only 4 patients (2 perimembranous VSDs with aneurysms, 1 Gerbode, and 1 postoperative VSD in a patient with CCHD) required the placement of 2 NitOcclud devices in the same defect due to persistence of a residual shunt, and 3 of these 4 double implantations were performed in a single catheterization. In addition, 1 patient (12kg, with heterotaxy syndrome and CCHD) required a 7-mm Amplatzer Vascular Plug type IV during the implantation over an 18/6-mm NitOcclud for the same reason.
Fluoroscopy time was significantly higher for both perimembranous VSDs without aneurysm and postoperative residual shunts (table 2). The total procedural time and failure rate of the implantation were also higher.
In 4 patients, additional shunts were also closed: 1 ductus, 2 VSDs/foramen ovale, and 1 anterior muscular VSD (7kg, with CCHD; using a 6 × 4-mm Amplatzer Ductal Occluder type II).
Of the 11 patients whose implantation failed, 4 had Laubry syndrome (with one of the aortic cusps close to the shunt),17 1 with VSD after inferior wall infarction (compassionate treatment due to heart failure and prior partial occlusion with an Amplatzer Ventricular Septal Defect Occluder), 4 perimembranous VSDs with aneurysmal tissue with a persistent unstable coil, and 2 perimembranous VSDs without aneurysmal tissues with a persistent shunt (of at least moderate degree). In another patient with perimembranous VSD without aneurysmal tissue (8 years old, 39kg), the occluder embolized the left pulmonary artery and was percutaneously removed 24hours after implantation.
The average follow-up period was 31.4 (0.6-59.9) months. No deaths or permanent rhythm disorders were associated with the implantation. Overall, the major complication rate was 6.7% (7 of 106). This percentage was higher for Gerbode-type VSDs and postoperative residual VSDs (table 2).
Over time, the principal complication was hemolysis, identified in up to 5.8% of patients (6 of 104); it appeared in the first 24 to 48hours after the implantation. It was severe in 4 patients, and 2 needed surgical treatment (table 4). A third patient with controlled chronic hemolysis underwent a heart transplant due to heart failure secondary to CCHD.
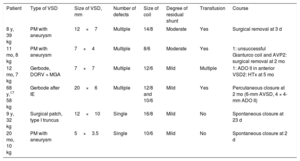
Description of patients with hemolysis
| Patient | Type of VSD | Size of VSD, mm | Number of defects | Size of coil | Degree of residual shunt | Transfusion | Course |
|---|---|---|---|---|---|---|---|
| 8 y, 39 kg | PM with aneurysm | 12×7 | Multiple | 14/8 | Moderate | Yes | Surgical removal at 3 d |
| 11 mo, 8 kg | PM with aneurysm | 7×4 | Multiple | 8/6 | Moderate | Yes | 1: unsuccessful Gianturco coil and AVP2: surgical removal at 2 mo |
| 12 mo, 7 kg | Gerbode, DORV + MGA | 7×7 | Multiple | 12/6 | Mild | Multiple | 1: ADO II in anterior VSD2: HTx at 5 mo |
| 68 y,17 58 kg | Gerbode after IE | 20×6 | Multiple | 12/8 and 10/6 | Mild | Yes | Percutaneous closure at 2 mo (6-mm AVSD, 4 × 4-mm ADO II) |
| 9 y, 32 kg | Surgical patch, type I truncus | 12×10 | Single | 16/8 | Mild | No | Spontaneous closure at 23 d |
| 20 mo, 10 kg | PM with aneurysm | 5×3.5 | Single | 10/6 | Mild | No | Spontaneous closure at 2 d |
ADO, Amplatzer Ductal Occluder; AVP, Amplatzer Vascular Plug, AVSD, Amplatzer Ventricular Septal Defect Occluder; DORV, double-outlet right ventricle; HTx, heart transplantation; IE, infectious endocarditis; MGA, malposition of the great arteries; PM, perimembranous; VSD, ventricular septal defect.
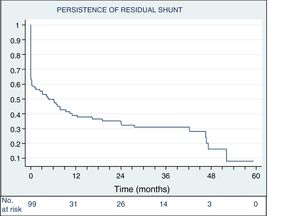
Four patients (3.8%) needed a second percutaneous procedure, 1 for hemolysis and 3 for significant residual shunt (figure 4).
In total, 9.6% of the patients (10 of 104) developed transient cardiac rhythm disorders. Two were transient AVBs: in 1 patient (21kg, with a perimembranous VSD without aneurysm) with third-degree AVB and significant residual shunt, the atrioventricular conduction normalized after device removal and surgical occlusion of the defect 24hours after the implantation18; the second patient (24kg, with Down syndrome and an aneurysmal perimembranous VSD) required periodic administration of atropine and treatment with oral corticosteroids for second-degree Mobitz type II AVB in the first 48 hours, with favorable progression to sinus rhythm after 6 months of follow-up. Another patient (10kg, with a CCHD) developed electromechanical dissociation during the arteriovenous loop closure; remission was achieved via the administration of adrenaline and fluids, with a subsequent successful coil implantation and incident-free follow-up. Nodular rhythm developed in 1 patient (56kg, with an aneurysmal perimembranous VSD) who underwent intraprocedural substitution of the initially applied coil (8/6 for a larger 10/6) to minimize residual shunt, which resolved after 4-day administration of corticosteroids. Junctional ectopic tachycardia occurred in 2 patients in the first week after implantation (12 kg, with Gerbode-type; and 16kg, with a perimembranous VSD), with remission after the administration of corticosteroids for 7 to 14 days and favorable progression 3 to 5 years after the implantation. Other self-limiting conditions in the first 48hours were 1 patient with premature supraventricular contractions, 1 patient with premature ventricular contractions, and 2 with intermittent nodular rhythm; all resolved without medical therapy.
We registered the onset of right bundle branch block without atrioventricular conduction delay in 3 patients, which persisted for 2 years of follow-up.
There were no cases of valve damage secondary to the implantation that required intervention. However, there was valve interference in 6.7% of patients (7 of 104): 2 with aortic regurgitation, 3 with new or aggravated tricuspid regurgitation, 1 mild double tricuspid lesion that spontaneously resolved after 10 months of follow-up, and 1 grade II mitral regurgitation (managed via intraprocedural substitution of a 14/8 coil for a 12/8 coil).
Other minor complications were 1 femoral arteriovenous fistula in the left lower limb, 1 femoral arterial thrombosis (repermeabilized with heparin), and 1 transient neuropathy of the right brachial plexus.
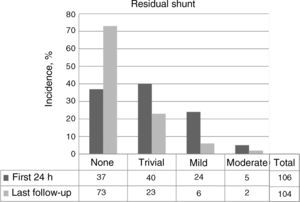
The rates of complete or almost complete closure 48 hours after the procedure and at last follow-up were 72.6% (77 of 106) and 92.3% (96 of 104), respectively (figure 5). The changes in possible residual shunts over time in patients with a successful implantation not requiring further intervention are shown in figure 6.
Since the first description of percutaneous VSD occlusion,2 the technique has spread and the field of application has expanded due to better experience and an improved variety of available devices. The peculiarity of these devices is that they have both a specific design and can be adapted for offlabel use.13,15,19 Furthermore, this approach become the preferred method in some institutions due to its advantages over surgical techniques9,20 (especially in Asia and for muscular VSDs).14,21
In perimembranous VSDs, the principal problem is the onset of permanent AVB, even years after the implantation. This complication is also associated with the surgical approach,22 but its current rate of AVB occurrence is estimated to be < 1%.23 A complete AVB rate of 1.6% (0.8% with permanent pacemakers) was recently recorded after percutaneous occlusion with a modified double-disc device (n = 1046).24
The ideal characteristics of occluders are that they are low profile and easy to use, adaptable to multiple forms of anatomical defects, and easy to remove and reconfigure. They should be able to close the defect in a reasonable amount of time without modifying or interfering with adjacent cardiac structures, thereby minimizing the risk of an atrioventricular conduction disorder.10
According to our study, the NitOcclud Lê VSD Coil fulfils most of the aforementioned characteristics and can be applied to a wide range of clinical conditions, achieving an overall success rate of 89%. It was most commonly indicated for small- to mid-sized aneurysmal perimembranous VSDs in asymptomatic school-aged patients with echocardiographic left-chamber dilatation. Patients with perimembranous VSDs without aneurysmal tissue or postoperative residual VSDs are more complicated from a technical point of view (longer fluoroscopy time and lower success rate) (table 2).
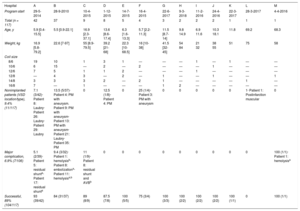
In relation to the literature (table 5),10–15 this study has recruited the largest number of patients and the widest variety of VSDs in terms of type (including ischemic) and location (including Gerbode).16 Very few studies refer to the percutaneous occlusion of Gerbode-type VSDs, and even fewer with the NitOcclud device.14,25 It is worth noting that 3 catheterizations were performed with the simultaneous and elective implantation of 2 coils on the same defect. We also included patients of a young age or low weight (6.8%, < 1 year; 8.6%, < 10 kg), generally in the context of postoperative VSDs in CCHD, where the procedure is particularly difficult.26 This could explain why our overall success rate (89%) is slightly lower than that of other studies and why the incidence of major complications (6.7%) and the fluoroscopy time (37 minutes) are slightly higher (table 4). In addition, our definition of major complications included percutaneous reinterventions and not only surgical procedures, in contrast to other series10,13 (which would decrease our complication rate to 2.8% [3 of 106]).
Previous studies of the use of the NitOcclud® Lê VSD Coil for VSD closure
| Authors | Type of VSD | Age, y/weight, kg | Successful, % | Fluoroscopy time/procedural time, min | Major complications | Other complications | Hemolysis | pAVB | RBBB | Residual shunt | Valve damage | Follow-up, mo |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chungsomprasong et al.12,a(2011; n=33) | PM=11DC=22 | 9.8 [1-29]/34.5 [10.1-83.2] | 93.9 | 21.9 [8.2-75)/88.2 [60-150) | No | — | No | No | — | n=5 (15.2%) at 6 mo | — | 8.1 [3-76.2] |
| Odemis et al.11(2014; n=20) | PM=20Aneurysm=19 | 7.3 [1.3-17)/25.7 [10-58) | 100 | 29.4 [13.8-67.4]/88.5 [40-180] | n=1 (5%) Sx | — | n=3 (15%) Sx=1 | No | — | n=3 (15%) at 90 d | No | 12.3 [2-22] |
| EUREVECO10(2017; n=111) | PM=81Aneurysm=48Muscular=31 | 8.4 (0.8-66.9)/28.8 (7.2-109) | 91.9 | 26.3 [7.5-86.3]/121.1 [15-278] | n=2 (1.9%) Sx | n=19 (18.5%) | n=3 (2.9%) Sx=1 | No | n=6 (5.9%) | n=3 (3.1%)at 1 y | TR=5AR=3 | 31.3 [24-48] |
| Nguyen et al.13,b(2017; n=71) | PM=71Aneurysm=47 | 16.8±14.4/31±18.7 | 97.2 | 27.1±12.4/92.7±36.5 | n=1 (1.4%) Sx | n=11 (14.5%) | n=5 (7.3%)Sx=0 | n=1 (1.4%)Sx=1 | No | n=4 (5.8%)at 1 y | AR=1 | 57.2±21.3 |
| El Shedoudy et al.14(2017; n=80) | PM with aneurysm=77Muscular=2Gerbode=1 | 5.3 (1.5-28)/17.2 (7.8-44) | 98.7 | 30.6 [26-39]/105 [86-130] | n=1 (1.3%) Pc | — | No | No | n=1 (1.3%) | n=2 (2.5%)at 1 y | No | 36 |
| Shrestha et al.15(2017; n=59) | PM=59 | 7.1 (0.8-28)/21.2 (6.4-93) | 97c | 22 [4.8-77.1]/60 [30-260]c | n=1 (1.4%) Sx | n=12 (9%) | No | No | — | n=10 (9.6%)c at 1 y | AR=1Sx | 12 |
| Solana-Gracia et al. (n=117) | PM=96Aneurysm=74Muscular=7Gerbode=6Postoperative residual=9 | 8.6 (0.4-69.2)/27.5 (5.8-97) | 89 | 34 [11.4-124]/133.5 [60-285] | n=7 (6.9%)Sx n=2Pc+Sx n=1Pc n=4 | n=24 (20.5%) | n=6 (5.8%)Sx=2 | No | n=3 (2.9%) | n=8 (degree ≥ mild)(7.7%) at 31 mo | TR=4AR=2MR=1 | 31.4 [0.6-59.9] |
AR, aortic regurgitation; DC, doubly committed; MR, mitral regurgitation; pAVB, permanent atrioventricular block; Pc, percutaneous; PM, perimembranous; RBBB, right bundle branch block; Sx, surgery; TR, tricuspid regurgitation; VSD, ventricular septal defect.
Unless otherwise indicated, the results are expressed mean ± standard deviation, or median [interquartile range].
The main factor determining the correct adaptation of the device to the interventricular septum, and thereby avoiding residual shunt and interference with the aortic valve, is the careful consideration of both the type of defect and the size of the coil. This makes transesophageal echocardiography essential, given that it provides detailed information on the hemodynamic defect: the presence of multiple apertures, the characteristics of the possible surrounding aneurysmal tissue, and the relationship with the aortic valve annulus. However, evaluation of the anatomical defect is more precise when performed with long-axis left ventriculography (60° left anterior oblique and 20° cranial angulation). The choice of coil aims to “fill” the hole generated by the VSD (especially if there is an aneurysmal sac) while avoiding interference with the aortic valve. Residual shunts are usually either a result of an incorrectly sized occluder (generally undersized) or an inadequate configuration of the device coils in relation to the anatomical defect. It should be remembered that there is a certain amount of random variability concerning occluder adaptation to the interventricular septum.
While the presence of a residual shunt permits hemolysis development, the magnitude of the shunt cannot be correlated with the degree of hemolysis. For this reason, and according to our experience, we recommend a strict approach to the presence of residual shunt, especially if it is either significant or periprosthetic. In these cases, it is advisable to attempt to reposition the device over the defect or to switch to a larger size. If this is not possible, the possible implantation should be considered of a second occluder in situ.
Hemolysis generally appears after 24 hours and, in some very exceptional cases, more than 2 weeks after implantation. The first therapeutic recommendation would be patient hydration and aspirin withdrawal. Percutaneous treatment should be reserved as a second-line option, with explantation and surgical occlusion of the VSD the last recourse.
In addition, no clear learning curve is visible from analysis of the experience of each hospital (in general, the first procedures were all supervised by a proctor) (table 3). Implantation failure and complication development were more strongly associated with the type of VSD and the individual characteristics of the patients. Due to its anatomy, we would not recommend this technique for treating a Laubry syndrome-type defect17 because the proximity of the coronary cusp (usually the right) to the defect interferes with the conformation of the coil on the defect.
This series is the first to describe the development of ectopic junctional tachycardia after NitOcclud implantation. Its onset was subacute and it showed a benign course.
In contrast to the self-expandable double-disc systems, the main advantage of this device is the marked reduction in the rate of permanent AVB and pacemaker need, because no clamping stress is exerted on the tissue around the defect. In our series, there were no such cases and there is only 1 patient in the literature with this complication.13
Similarly, there were no cases of postoperative infectious endocarditis, described in the literature as a rare complication.27
LimitationsGiven the methodology of our study, we could not ascertain either the total number of patients who would benefit from this treatment or, in contrast, how many have already undergone other treatments (percutaneous or surgical), making it impossible to compare outcomes among different techniques.
CONCLUSIONSDue to the unusual nature and reduced number of patients with congenital heart disease, collaboration between institutions is vital to obtain results on the development of techniques for highly specific treatments.28. This work concludes that the NitOcclud Lê VSD Coil can be successfully used for a wide anatomical spectrum of VSDs without inducing permanent AV conduction disorders. The incidence of hemolysis is very low and does not affect the indication for the procedure. However, the persistence of residual shunt should be minimized and any patients identified to have such a shunt should be periodically revised.
- -
The NitOcclud Lê VSD Coil was introduced in 2010 with a new and specific design for VSD closure.
- -
It was developed with the aim of minimizing AVB onset (the principal and potentially fatal complication of the double-disc systems that had been used until then).
- -
Previously published series on its use are limited in terms of size and follow-up.
- -
However, previous reports highlight its safety and the significant reduction in permanent AV conduction disorders.
- -
Nevertheless, the risk of hemolysis in patients with residual shunt must be noted.
- -
Our study compiles the Spanish experience with this technique.
- -
It represents the largest and most heterogeneous series in terms of patient characteristics (weight < 10 kg, n = 12) and VSD type and anatomical location.
- -
A more complicated approach is required for the occlusion of perimembranous VSDs without aneurysmal tissue or postoperative residual VSDs.
- -
We advise against using this technique in patients with Laubry syndrome because the unfurling of the coil can interfere with the prolapsed aortic cusp.
- -
The persistence of a residual shunt with a higher-than-mild degree should be avoided or that located between the device and the margins of the VSD.
J.L. Zunzunegui Martínez and J.M. Velasco Bayón are proctors of PFM Medical.
We thank the Task Force for Pediatric Catheterization of the Spanish Society of Pediatric Cardiology and Congenital Heart Disease.