The purpose of this article is to present the results obtained from heart transplantation since this therapeutic modality first began to be used in Spain in May 1984.
MethodsA descriptive analysis was performed of all heart transplantations performed until 31 December 2011.
ResultsThe total number of transplantations is 6528. The average clinical profile of the Spanish heart transplantation patient in 2011 was that of a 53-year-old male who had been diagnosed with nonrevascularizable ischemic heart disease accompanied by severely depressed ventricular function and poor functional status. The implanted heart was typically from a 38-year-old donor who had died from brain hemorrhage. The average waiting list time was 122 days. Mean survival time has progressively increased over the years. For the overall series, the probability of survival at 1, 5, 10, and 15 years was 77%, 66%, 53%, and 39%, respectively, whereas over the past 5 years the probability of survival at 1 and 5 years was 80% and 73%, respectively. The most frequent cause of death was acute graft failure (16%), followed by infection (15.6%), the combination of graft vascular disease and sudden death (14%), tumors (12.3%) and acute rejection (7.7%).
ConclusionsThe survival rates obtained in Spain from heart transplantation, especially in recent years, place heart transplantation as the treatment of choice in irreversible heart failure patients without other established medical or surgical options.
Keywords
This article is the annual update analysis, published since 1991, describing the results of heart transplantation (HT) activity conducted in Spain between the first such procedure, performed in May 1984, and December 31 of the year prior to publication.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22
This Registry includes data on all HTs performed by all teams at all centers in Spain (Appendix) through 2011. It is, therefore, an accurate account of the status of HT in our country. The report's reliability is founded on the nationwide use of a single database constructed on mutually agreed principles, which standardizes variables and the possible responses.
Methods Patients and CentersNineteen centers have supplied the registry with data (Table 1), although only 18 are currently carrying out transplantations.
Table 1. Spanish Heart Transplantation Registry Collaborators 1984-2011 Participating Centers
| 1. Hospital de la Santa Creu i Sant Pau, Barcelona |
| 2. Clínica Universitaria de Navarra, Pamplona |
| 3. Clínica Puerta de Hierro, Majadahonda, Madrid |
| 4. Hospital Marqués de Valdecilla, Santander |
| 5. Hospital Reina Sofía, Córdoba |
| 6. Hospital Universitario y Politécnico La Fe, Valencia |
| 7. Hospital Gregorio Marañón, Madrid |
| 8. Fundación Jiménez Díaz, Madrid |
| 9. Hospital Virgen del Rocío, Sevilla |
| 10. Hospital 12 de Octubre, Madrid |
| 11. Hospital Universitario A Coruña, A Coruña |
| 12. Hospital de Bellvitge, L’Hospitalet de Llobregat, Barcelona |
| 13. Hospital La Paz, Madrid |
| 14. Hospital Central de Asturias, Oviedo |
| 15. Hospital Clínic, Barcelona |
| 16. Hospital Virgen de la Arrixaca, El Palmar, Murcia |
| 17. Hospital Miguel Servet, Zaragoza |
| 18. Hospital Clínico, Valladolid |
| 19. Hospital Vall d’Hebron, Barcelona |
Ordered according to first transplantation.
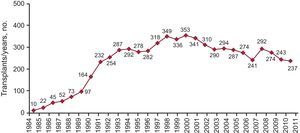
In more than 25 years of transplantation activity, more than 6528 HTs have been performed. Of these, 94% were isolated orthotopic transplants. Figure 1 displays the HT frequency distribution by year. Table 2 presents the distribution by type of HT procedure.
Figure 1. Number of transplants per year.
Table 2. Spanish Heart Transplantation Registry Collaborators 1984-2011 by Type of Procedure
| De novo heart transplantations | 6202 |
| Heart retransplantations | 191 |
| Combined transplants | |
| Heart-lung | 77 |
| Heart-kidney | 50 |
| Heart-liver | 8 |
| Total | 6528 |
The database includes 175 clinical variables with data on recipients, donors, surgery, immunosuppression, and follow-up. Each year, the centers send data to the Registry Director, who organizes the statistical methodology with the company hired to perform the analysis (currently ODDS, SL). An audit of the centers is organized periodically to verify registry data. The audit is carried out by an independent company which randomizes the centers and the HTs, extracts a representative sample of information, and verifies the reliability of the data submitted.
In 2008, the Registry was presented to and approved by the Committee for Biomedical Research Ethics of the Hospital Universitario La Fe, Valencia. On the other hand, the Registry is in the process of being registered in the Spanish Ministry of Health, Social Services and Equality to guarantee fulfillment of the Spanish Data Protection Law 15/1999. Furthermore, the database is expected to be available online by 2013.
StatisticsVariables are presented as the mean±the standard deviation and percentage. Survival curves have been calculated using the Kaplan-Meier test, and compared using the long rank test. The statistical significance is reached when P<.005. Survival data analysis has not included retransplantations or combined transplantations.
Results Heart Transplant Patient ProfileThe average clinical profile of HT recipients in Spain is that of a 53-year-old male diagnosed with ischemic heart disease or idiopathic dilated cardiomyopathy, with blood group A or O. Table 3 shows the clinical profile of HT recipients distributed by age. Retransplantation patients are analyzed independently.
Table 3. Spanish Heart Transplantation Registry Collaborators 1984-2011 Clinical Profile of Recipients, Categorized as Pediatric, Adult, or Retransplantation
| <16 years | ≥16 years | Retransplantation | |
| Number | 288 | 5694 | 155 |
| Males, % | 62.5 | 81.7 | 78.1 |
| Age, years | 6 (5.7) | 53.2 (11.9) | 50.1 (14) |
| BMI | 15.7 (4.9) | 25.3 (4) | 25 (4.1) |
| Initial etiology, % | IHD: 1.4 | IHD: 34.4 | GVD: 34.6 |
| iDMC: 34 | iDMC: 29.8 | AGF: 16.3 | |
| VHD: 0.7 | VHD: 8.9 | ARe: 11.1 | |
| CHD: 39.7 | CHD: 1.5 | Other: 38 | |
| Other: 24.1 | Other: 25.4 | ||
| Blood type | |||
| A | 54 | 49 | 57 |
| B | 7 | 9 | 8 |
| AB | 4 | 5 | 5 |
| 0 | 35 | 38 | 30 |
| FC III-IV/IV | 68 | 62 | 69 |
| Creatinine>2 mg/dL | 3 | 5 | 27 |
| mPAP, mmHg | 30 (13) | 30 (11) | 27 (9) |
| PVR, WU | 3 (2) | 2 (2) | 2 (1) |
| Bilirubin>2 mg/dL | 18 | 16 | 19 |
| GOT/GPT×2, mg/dL | 22 | 26 | 28 |
| Diabetes mellitus ID | 1 | 14 | 18 |
| HBP | 2 | 28 | 42 |
| Hypercholesterolemia | 3 | 37 | 44 |
| COPD, moderate-severe | 2 | 11 | 7 |
| Previous HS | 28 | 25 | 100 |
| Inotropic therapy | 68 | 34 | 53 |
| Mechanical ventilation | 31 | 10 | 31 |
AGF, acute graft failure; ARe, acute rejection; BMI, body mass index; CHD, congenital heart disease; COPD, chronic obstructive pulmonary disease; FC, functional class; GOT (AST), aspartate aminotransferase; GPT (ALT), alanine aminotransferase; GVD, graft vascular disease; HBP, high blood pressure; HS, heart surgery; ID, insulin dependent; iDCM, idiopathic dilated cardiomyopathy; IHD, ischemic heart disease; mPAP, mean pulmonary artery pressure; PVR, pulmonary vascular resistances; VHD, valvular heart disease; WU, Wood units.
Values are given as mean (standard deviation) and percentages.
Combined transplants are not included.
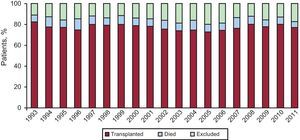
In 2011, the mortality of patients in the waiting list was 5%. After being added to the waiting list, 20% of the patients were excluded from HT. Figure 2 represents the annual percentage of patients included on the waiting list who received HT, were excluded from the list, or passed away before receiving a transplantation.
Figure 2. Patient outcomes once included on heart transplant waiting list.
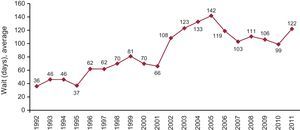
The mean time recipients had to wait for HT in 2011 was 122 days. Figure 3 shows the evolution of the wait days throughout the last 20 years.
Figure 3. Year-by-year evolution of mean days on waiting list for heart transplantation recipients.
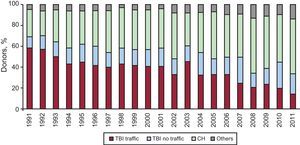
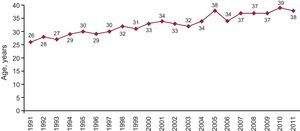
Cause of Death and Mean Donor AgeMost of the donor hearts come from individuals who died of cerebral hemorrhage. The mean age in 2011 was 38 years, as displayed in Figure 4, Figure 5.
Figure 4. Year-by-year evolution of causes of heart transplant donor deaths. CH, cerebral hemorrhage; TBI, traumatic brain injury.
Figure 5. Year-by-year evolution of the mean age of heart transplant donors.
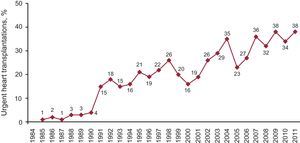
Emergency TransplantationThe percentage of indicated emergency transplantations in 2011 was 38%. Figure 6 shows the evolution of this HT option throughout the years.
Figure 6. Year-by-year evolution of emergency transplantation percentage.
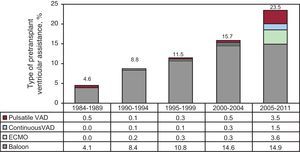
Ventricular Assist DevicesThe percentage of patients transplanted with assist devices has increased over time. Within the last 7 years, it reached 24%. The distribution by time periods, as well as by type of ventricular assist device, can be seen in Figure 7.
Figure 7. Distribution of ventricular assist device types used prior to transplantation, by time periods. ECMO, extracorporeal membrane oxygenation device; VAD, ventricular assist devices.
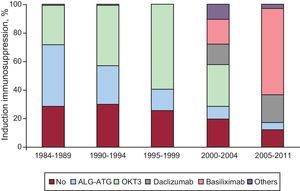
ImmunosuppressionMost patients who received HT in Spain are given induction immunosuppressive treatment. The different drugs used and distribution by time periods are displayed on Figure 8.
Figure 8. Induction immunosuppression. Drugs administered.
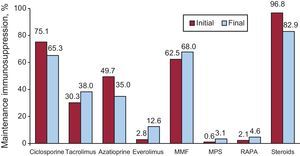
De novo maintenance immunosuppressive treatment and changes made during the recipients’ evolution appear in Figure 9.
Figure 9. Maintenance immunosuppression variations in clinical course by drug type. Immunosuppression at transplantation and at end of follow-up. MMF, mycophenolate mofetil; MPS, mycophenolate sodium; RAPA, rapamycin.
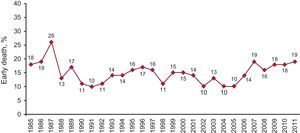
SurvivalEarly mortality (within 30 days from HT) was 19% in 2011, as seen in Figure 10. This particular mortality is slightly higher than the mean of the previous 5 years (17%).
Figure 10. Year-by-year percentage evolution of early deaths (first 30 days).
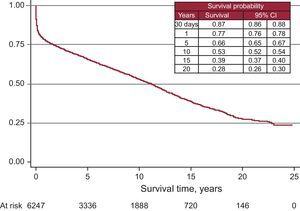
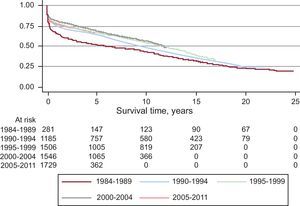
After incorporating the survival data from 2011 to the data from the previous years, we obtained an actuarial survival probability of 87% for the first month. For 1, 5, 10, 15, and 20 years we obtained actuarial survival probabilities of 77%, 66%, 53%, 39%, and 28%, respectively (Figure 11). Survival per time period improved in the later years, with a survival probability of 80% at 1 year and 73% after 5 years (Figure 12).
Figure 11. Survival curve for the entire series. 95%CI, 95% confidence interval.
Figure 12. Survival curve by time periods.
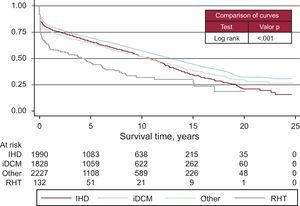
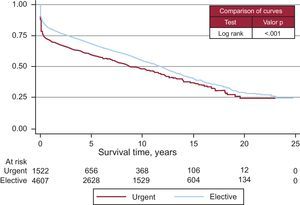
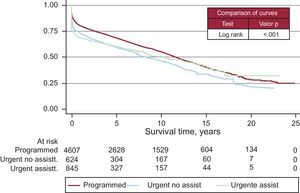
Survival curves varied according to the etiological reason for HT (Figure 13). The degree of urgency also influenced the survival probability (Figure 14). Nonetheless, there were no differences among patients urgently transplanted with assistance, whether this was with intra-aortic balloon, membrane oxygenation, or ventricular assist device or without it (Figure 15).
Figure 13. Survival curves according to etiology indicating transplantation. iDCM, idiopathic dilated cardiomyopathy; IHD, ischemic heart disease; RHT, retransplantation.
Figure 14. Survival curve by degree of emergency.
Figure 15. Survival curve by degree and type of emergency. Statistical significance between urgent and not urgent degree. No differences between types of emergency (with and without assistance). Assistance includes: intra-aortic balloon counterpulsation, extracorporeal membrane oxygenator, and pulsatile or continuous ventricular assistance. assist., assistance.
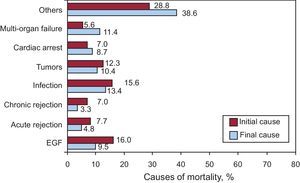
Causes of DeathThe most frequent cause of death was early graft failure (16%), followed by infection (15.6%), the combination of graft vascular disease and sudden death (14%), tumors (12.3%), and acute rejection (7.7%) (Figure 16).
Figure 16. Causes of Death. Initial and final cause leading to death. EGF, early graft failure.
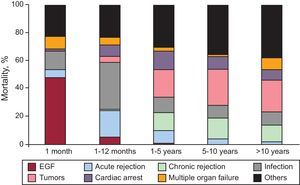
By distributing the causes of mortality across several time periods, we were able to appreciate that they vary. In the first month, early graft failure was the predominant cause of death. Between the first month and the first year the main causes were infection and transplantation rejection. Tumors and the combination of chronic rejection with sudden death were the predominant causes after the first year. In Figure 17 we can observe the causes of death distributed by time periods.
Figure 17. Causes of death by time since transplant. EGF, early graft failure.
DiscussionAfter almost 30 years of HT development in Spain and with more than 6500 HT performed, we are able to say that this therapeutic modality can be offered to all the population, guaranteeing levels of knowledge, control, quality standards, and survival similar or superior to those of other developed countries and the rest of the world. This can be confirmed if we compare our results with those of the annual publication of the International Society for Heart and Lung Transplantation Registry.23, 24, 25, 26
One of the greater advantages of the Spanish Registry of Heart Transplantation is that we have elaborated a homogeneous database among all the Spanish Transplantation teams, agreeing about all possible responses. Each year, all the teams update their database and send them to the registry director, who merges them and then forwards them to an independent statistical company for the appropriate analysis. This method is considered to provide reliable results and avoid errors, which are very common in nonhomogeneous databases. In 2007, the number of analyzed variables per patient reached 175. In addition, aiming for higher data quality and reliability, we mean to continue with the audit of centers through independent companies that guarantee the validity of the data.
Currently, 18 centers have transplanting activities. In Spain, transplantation teams are concerned because HT centers are being authorized without an adequate analysis of the necessary requirements. This is due to the clear decreasing trend of optimal donor numbers in Spain, which consequently causes the number of HTs/number of centers correlation to decrease. The reduced number of HTs performed causes, on one side, the underutilization of hospital resources that are prepared and available for multiple functions and, on the other, lengthens the learning process needed to achieve optimal results. The only benefit of the new centers to the patient is that he/she may stay in the same location, although this is only a benefit when there is an authorized center already in place at the location. Since health authorities have decided to open more centers, they must be the ones to evaluate whether a true optimization of resources in “times of [economic] crisis” is occurring.
Last year, the number of HTs performed decreased again due to the progressive trend of donor decrease (237 in 2011 compared to 243 in 2010). There is no single explanation for this decline, but it seems evident that lower mortality from traffic-related head injury along with better control and management of patients with multiple trauma units may be a cause. When the number of donors decreases, the possibilities of HTs decrease and the number of patients on the waiting list grows. Therefore, the proportion of patients with advanced heart failure who, once on the waiting list, cannot receive a transplant and are removed from the list (by death or deterioration) reaches 20%. Transplantation teams, aware of this problem, have attempted to expand the range of possible donors by expanding the donation criteria. Even so, the average age of donors shows very little variation (38 years in 2011 compared to 39 years in 2010).
The time that patients must wait for a compatible heart increased significantly, from 99 days in 2010 to 122 days in 2011. In previous years the wait had decreased due to the higher numbers of emergency transplantations. However, given that the time it takes to find a compatible organ for emergency transplantations is increasing, it is very likely that the waiting time will increase progressively in the next years.
The clinical profile of patients has not changed in the last few years. The patients have been divided in three groups (pediatrics, adults, and retransplantations), given that they have different clinical characteristics. Thus, pediatric patients receive transplantations due to congenital heart disease or idiopathic dilated cardiomyopathy; they have higher pulmonary resistance and absence of cardiovascular risk factors. Retransplantations are usually caused by graft vascular disease, with more organ deterioration and more risk factors. This, rather than the fact that it is a second transplantation, may contribute to a worse prognosis.
Emergency HT is subject to controversy because certain characteristics (recipients in worse clinical condition, not ideal donors, and longer ischemic times) of these interventions bring forth a worse prognosis that when the HT can be programmed. In these last years, the number of emergency transplantations also increased (38% in 2011 compared to 34% in 2010). The percentage of patients that are counted as urgent differs from area to area and changes noticeably through the years. The reasons for these changes or for the geographical distribution are unclear, although we suspect that a low number of donors and better maintenance of critical patients (ventricular assist) increase the odds of emergency transplantations being performed. The necessity of an emergency transplantation has been questioned given that the results are clearly not good. However, transplantation teams consider that the option must exist, if in a controlled manner. As recommended by the recent European guidelines on heart failure, in order to guarantee the survival of the patient we must keep in mind that the patient must be stabilized before HT is indicated. Also, HT should not be considered treatment for acute unstable heart failure27 because it takes too long to find a donor even with this degree of emergency, among other reasons.
The number of patients that reach HT with some form of ventricular assistance has increased significantly, especially in the last 5 years. The intra-aortic counterpulsation balloon is still the most widely used, although its use has not increased in the last 5 years. The use of extracorporeal membrane oxygenators and pulsatile devices have significantly increased. More than half of the emergency transplantation patients in the last 5 years carried some sort of ventricular assistance previous to transplantation. These devices are crucial for the maintenance and stabilization of patients with chronic heart failure. Therefore, it is advised that all transplantation teams have access to them to be used on the most critical patients. Furthermore, they are of great utility in case of a fatal graft failure immediately after transplantation. This complication is becoming more frequent because of worsened conditions of recipients, nonoptimal donors, and the longer ischemic times that come with the degree of emergency and the distance to the organ.
In the majority of HTs, immunosuppression induction has been used. The most-used treatment since the beginning has been antilymphocyte antibodies, OKT3. However, the most widely used in the last 5 years are interleukin-2 antagonists, which represent 85% of the transplantations performed. The maintenance immunosuppressive therapy used is called triple association: tacrolimus versus cyclosporine, azathioprine versus mycophenolate, and mofetil versus steroids. However, while the patient progresses it is usual to introduce other immunosuppressive drugs such as rapamycin, everolimus, mycophenolic acid, and more recently, sustained-release tacrolimus. The administration of everolimus has increased the most. It is administered to 2.8% of patients at the start of transplantation but is included in up to 12.6% of transplantations when renal dysfunction, tumors, or graft vascular disease concur.
Early mortality rose from 18% in 2010 to 19% in 2011. Over the last 4 years this trend has been increasing. The increase may be related to a greater number of emergency transplantations and to the use of ventricular assists, with which the patients reach HT in even more critical condition. The early period after transplantation is the most important when it comes to increasing survival. The survival curve stabilizes durng the first months after HT.
General survival reveals a clear trend towards improvement. Nevertheless, as expected, the number of patients incorporated to the registry every year represents a number lower than the total, and thus the probability of big changes in a year is very unlikely. This makes the analysis of survival by time periods a better display. In the last few years, survival has increased significantly as compared to previous years. Nonetheless, there is an “inactivity” in the survival curve that has been attributed to the worsened clinical situation of recipients and to the fact that organs are less optimal and have longer ischemic time. However, even in high-risk HT groups the survival is much greater than in patients with advanced heart failure without transplantation.
The cause of transplantations is obviously related to survival. Patients diagnosed with idiopathic dilated cardiomyopathy have a higher survival than those transplanted for other causes, due to their younger age and lower prevalence of cardiovascular risk factors.
The most frequent cause of death was early graft failure (16%), followed by infection (15.6%), the combination of graft vascular disease and sudden death (14%), tumors (12.3%), and acute rejection (7.7%). However, the actual cause of death is usually related to time from the HT, so that for the first month the most common cause of death is graft failure. From the first month until the first year, the main causes are infection and rejection. In subsequent periods, the most common cause of death is sudden death combined with chronic rejection and tumors. The observed distribution of causes of death has not changed in recent years, and should make us reflect on the need to achieve a “balance” in administering immunosuppression because death due to failure in preventing rejection is 7.7%, while death directly related to excessive immunosuppression (infection and tumors) is 28.2% of mortality cases.
ConclusionsTransplantation teams must consider donors with expanded criteria in order to offer this treatment to most patients with advanced heart failure and to prevent their poor prognosis.
Survival rates of the Spanish Heart Transplantation Registry are similar to other records. However, efforts should be increased to improve the likelihood of survival during the early period, which will result in significant overall improvement.
Ventricular assistance has boomed. These devices allow recipients to maintain appropriate conditions until the availability of a compatible organ. However, because sometimes the wait time can be weeks, it becomes necessary to have medium- and long-term ventricular assist devices available to prevent further deterioration of the patient and to keep him or her in optimal condition for transplantation.
There is still a large imbalance between the complications that immunosuppression prevents (rejection) and the ones that it favors (tumors, infection). In the coming years these problems must be addressed and immunosuppression must be customized to certain patient characteristics.
FundingStatistical analysis was carried out by ODDS, SL sponsored by an unconditional grant from Novartis Transplantation.
Conflicts of interestNone declared.
Acknowledgements
This year, elections will take place to choose a new Director of the Spanish Heart Transplantation Registry, an office that I have held since 1997. Throughout this time, I have attempted to perform the duties of this position with pride and honor. I wish to express my gratitude for all I have learned but, above all, for the enormous satisfaction of seeing that all the Spanish heart transplant teams have confided in me, sending me, year after year, the data concerning their transplantation procedures. Thank you all very much! (in words of the first author, Luis Almenar).
Appendix. Spanish Heart Transplantation Registry Collaborators 1984-2011| Clínica Puerta de Hierro Majadahonda, Madrid | Manuel Gómez-Bueno, María D. García-Cosío, Pablo García-Pavía, Luis Alonso-Pulpón |
| Hospital Universitario y Politécnico La Fe, Valencia | Luis Martínez-Dolz, Ignacio Sánchez-Lázaro, Mónica Cebrián |
| Hospital Universitario A Coruña, A Coruña | María J. Paniagua-Martín, Eduardo Barge-Caballero, Raquel Marzoa-Rivas, Zulaika Grille-Cancela |
| Hospital Gregorio Marañón (adults), Madrid | Juan Fernández-Yáñez, Adolfo Villa, Yago Sousa, Iria González, Manuel Martínez-Sellés |
| Hospital Reina Sofía, Córdoba | Amador López-Granados, Juan Carlos Castillo |
| Hospital Marqués de Valdecilla, Santander | José Antonio Vázquez de Prada, Manuel Cobo |
| Hospital 12 de Octubre, Madrid | María J. Ruiz, Pilar Escribano, Miguel A. Gómez, Marta Paradina |
| Hospital de la Santa Creu i Sant Pau, Barcelona | Vicenç Brossa, Sonia Mirabet, Laura López, Josep Padró |
| Hospital Virgen del Rocío, Sevilla | José Manuel Sobrino, Alejandro Adsuar |
| Hospital de Bellvitge, L’Hospitalet de Llobregat, Barcelona | Josep Roca, José González-Costello |
| Clínica Universitaria de Navarra, Pamplona | Beltrán Levy, Rafael Hernández |
| Hospital Clínic, Barcelona | Montserrat Cardona, Marta Farrero, M. Ángeles Castel |
| Hospital Central de Asturias, Oviedo | Beatriz Díaz |
| Hospital Gregorio Marañón (children), Madrid | Enrique Maroto, Constancio Medrano |
| Hospital Virgen de la Arrixaca, El Palmar, Murcia | Iris Garrido |
| Hospital Miguel Servet, Zaragoza | María L. Sanz, Ana Portolés |
| Hospital Clínico, Valladolid | Javier López-Díaz, Amada Recio |
| Hospital La Paz, Madrid | Daniel Borches, Luz Polo, Carlos Labrandero, Lucía Deiros |
| Hospital Vall d’Hebron, Barcelona | Ferran Gran, Raúl Abella |
Corresponding author: Hospital Universitario y Politécnico La Fe, Avda. Bulevar Sur s/n, 46026 Valencia, Spain. lualmenar@gmail.com