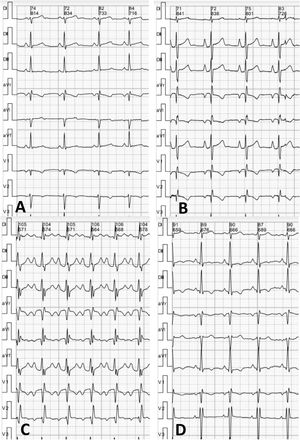
Pectus excavatum (PE) was previously considered to be a relatively common esthetic malformation; however, in the past decade it has been acknowledged to potentially affect cardiac function due to external compression on the right chambers of the heart.1 The protocol used for risk stratification and to assess preoperative eligibility usually includes functional heart studies such as exercise stress echocardiography (ESE).2 Electrocardiography (ECG) performed during ESE, using 12 thoracic leads, is often the only test available for these patients. However, there is scant information on its characteristics. Our aim was to analyze the ECG characteristics of patients with PE who underwent ESE as part of an eligibility assessment prior to surgery.
This retrospective, cross-sectional, observational study included patients diagnosed with PE who were assessed at an institution specializing in thoracic wall malformations and who had an indication for ESE to evaluate eligibility for a specific treatment. Patients were excluded if their ECG information was incomplete or of poor quality, if they had known lung or cardiovascular conditions, if there was a history of prior thoracic surgery, if they were currently receiving vacuum bell therapy, or if they did not grant informed consent for the use of their data (habeas data). The control group comprised young healthy participants with medical evaluations prior to participating in recreational amateur sports who showed no evidence of heart disease or thoracic malformations and who underwent ESE based on the same methodology as the PE patients. All procedures complied with the ethical standards of the teaching and research committee and with the Declaration of Helsinki of 1964 and its subsequent addenda. All patients underwent ESE using a bicycle in the supine position, based on a modified Astrand protocol.
The ECG analysis was performed by an experienced cardiologist blinded to the patients’ clinical characteristics and the results of their other studies. The following parameters were analyzed: type of heart rhythm, heart rate, PR interval duration, axis, P-wave voltage and duration, prevalence of an “Eiffel tower” P-wave morphology (figure 1), defined as voltage> 2mm, peaked shape with a narrow base (duration <90ms), QRS complex duration and axis in frontal plane, signs of right ventricular hypertrophy, presence and type of conduction disorder, presence and type of ventricular repolarization impairment as per conventional criteria, and frontal axis of T wave.
Between August 2012 and May 2018, 326 patients were diagnosed with PE on ESE at our hospital; 80 of these patients were included in the study, based on the inclusion and exclusion criteria (mean age, 17.2±6.6 y). The control group included 31 participants (mean age, 20.7±6.0 y). Patients with PE showed higher P-wave voltage in thoracic lead II (1.94±0.6 vs 1.68±0.5mm; P=.03). Patients with PE showed a high prevalence of “Eiffel tower” P waves (PE vs control, 66% vs 13%; P<.0001), longer PR interval (156.0±15.6 vs 148.1±18.9ms; P=.03), and higher prevalence of right bundle-branch block (34% vs 13%; P=.05). While no patients exhibited ST-segment downsloping, some did show abnormal T waves, specifically negative waves in thoracic leads II, III, and aVF (6% vs 0%; P=.008). Table 1 contains a detailed analysis of the electrocardiographic findings.
Demographic data and thoracic electrocardiography findings in patients with pectus excavatum and in the control group
| PE (n=80) | Control (n=31) | P | |
|---|---|---|---|
| Demographic data | |||
| Age, y | 17.2±6.6 | 20.7±6.0 | .02 |
| Men | 75 (94) | 19 (61) | <.0001 |
| Electrocardiography results | |||
| Ectopic atrial rhythm | 3 (4) | 1 (3) | .89 |
| Heart rate, bpm | 83.2±14.0 | 84.6±15.2 | .65 |
| PR, ms | 156.0±15.6 | 148.1±18.9 | .03 |
| Frontal P axis,° | 59.6±16.4 | 60.0±16.5 | .91 |
| Frontal P voltage, mm | 1.94±0.6 | 1.68±0.5 | .03 |
| P duration, ms | 89.4±8.9 | 87.7±9.2 | .39 |
| “Eiffel tower” P waves | 53 (66) | 4 (13) | <.0001 |
| QRS, ms | 92.1±13.2 | 95.8±14.8 | .21 |
| Frontal axis,° | 72.4±21.8 | 67.1±20.4 | .25 |
| RV hypertrophy | 3 (4) | 1 (3) | .15 |
| Blocks | .050 | ||
| RBBB | 26 (33%) | 4 (13%) | |
| LAFB | 0 | 1 (3%) | |
| RBBB+LAFB | 1 (1%) | 0 | |
| LBBB | 0 | 0 | |
| ST segment | .75 | ||
| Early repolarization | 8 (10) | 2 (7) | |
| Inferior ST-segment depression | 1 (1) | 0 | |
| Precordial ST-segment depression | 0 | 0 | |
| Precordial and inferior ST segment | 1 (1) | 1 (3) | |
| T wave | .008 | ||
| Abnormal FP | 5 (6) | 0 | |
| Abnormal HP | 0 | 0 | |
| Abnormal FP and HP | 0 | 3 | |
| Frontal T-wave axis,° | 47.9±21.6 | 50.7±16.9 | .52 |
FP, frontal plane; HP, horizontal plane; LAFB, left anterior fascicular block; LBBB, left bundle-branch block; P, P wave; PE, pectus excavatum; PR, duration of the PR interval; QRS, QRS complex; RBBB, right bundle-branch block; RV, right ventricle.
Regarding echocardiography variables, PE patients with “Eiffel tower” P waves had lower tricuspid annular plane systolic excursion (TAPSE, 18.8±4.5 vs 21.6±4.6mm; P=.012), smaller tricuspid annulus diameter (10.0±4.9 vs 13.3±6.2mm/m2; P=.010), and a higher prevalence of paradoxical septal motion (79% vs 56%; P=.027) and diastolic dysfunction during exertion (59% vs 33%; P=.033) than those with normal P waves.
This study identified distinctive electrocardiography findings for PE patients, such as greater voltage and morphological P-wave abnormalities, longer PR intervals, and prevalence of right bundle-branch block and T-wave abnormalities in the inferior leads.
In addition to higher voltage, 66% of PE patients had peaked P-wave morphology with a narrow base in thoracic lead II (“Eiffel tower” P wave). Although the causes were not analyzed, the increased voltage and the P-wave morphology at least indicate functional impairment, presumably as a result of compression caused by the thoracic deformation. Compared with patients with PE and normal P waves, those who had “Eiffel tower” P waves had reduced right ventricular systolic function, extrinsic compression of the atrioventricular groove, and a higher prevalence of indicators of impaired ventricular filling.
Nevertheless, although its prevalence was low, the identification of negative T waves in inferior thoracic leads could indicate underlying right ventricular function impairment.3
Electrocardiographic tracing from multiple locations of the thorax has been studied in various situations.4,5 In our setting, thoracic ECG obtained during ESE was often the only ECG available for PE patients other than ECG performed as part of a preoperative assessment.
The electrocardiographic abnormalities described may have multifactorial underlying mechanisms, and it is difficult to attribute these abnormalities to a specific variable.6 Prospective studies such as standard ECG should be undertaken to corroborate our findings.
As a limitation, demographic differences in sex and age could affect the results when comparing the control group with the PE patients.
In conclusion, PE patients often exhibit greater voltage and peaked “Eiffel tower” P-wave morphology, longer PR intervals, and higher prevalence of right bundle-branch block and negative T waves in inferior leads compared with a control group. Therefore, ECG could be an affordable and widely available tool for detecting potential cardiovascular conditions in PE patients.
FUNDINGThis study received no funding.
AUTHORS’ CONTRIBUTIONSC.A. Ingino: concept and design, data analysis, and interpretation; writing of the article; final approval; acceptance of responsibility for all aspects of the article and for researching and resolving any issue regarding the accuracy and truthfulness of any part of the study. I. Raggio: data collection, analysis, and interpretation; critical review of the intellectual content; final approval; acceptance of responsibility for all aspects of the article and for researching and resolving any issue regarding the accuracy and truthfulness of any part of the study. L. Toselli: data collection; critical review of the intellectual content; final approval; acceptance of responsibility for all aspects of the article and for researching and resolving any issue regarding the accuracy and truthfulness of any part of the study. J. Farina: data collection, critical review of the intellectual content; final approval; acceptance of responsibility for all aspects of the article and for researching and resolving any issue regarding the accuracy and truthfulness of any part of the study. G. Bellia-Munzón: concept and design; critical review of the intellectual content; final approval; acceptance of responsibility for all aspects of the article and for researching and resolving any issue regarding the accuracy and truthfulness of any part of the study. M. Martínez-Ferro: concept and design; critical review of the intellectual content; final approval; acceptance of responsibility for all aspects of the article and for researching and resolving any issue regarding the accuracy and truthfulness of any part of the study.
CONFLICTS OF INTERESTNone.