Spontaneous coronary artery dissection (SCAD) is a rare cause of acute myocardial infarction (AMI). We sought to compare the results on in-hospital mortality and 30-day readmission rates among patients with AMI-SCAD vs AMI due to other causes (AMI-non-SCAD).
MethodsRisk-standardized in-hospital mortality (rIMR) and risk-standardized 30-day readmission ratios (rRAR) were calculated using the minimum dataset of the Spanish National Health System (2016-2019).
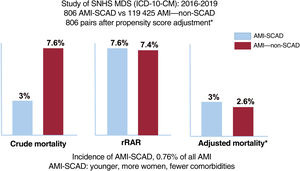
ResultsA total of 806 episodes of AMI-SCAD were compared with 119 425 episodes of AMI–non-SCAD. Patients with AMI-SCAD were younger and more frequently female than those with AMI–non-SCAD. Crude in-hospital mortality was lower (3% vs 7.6%; P<.001) and rIMR higher (7.6±1.7% vs 7.4±1.7%; P=.019) in AMI-SCAD. However, after propensity score adjustment (806 pairs), the mortality rate was similar in the 2 groups (AdjOR, 1.15; 95%CI, 0.61-2,2; P=.653). Crude 30-day readmission rates were also similar in the 2 groups (4.6% vs 5%, P=.67) whereas rRAR were lower (4.7±1% vs 4.8%±1%; P=.015) in patients with AMI-SCAD. Again, after propensity score adjustment (715 pairs) readmission rates were similar in the 2 groups (AdjOR, 1.14; 95%CI, 0.67–1.98; P=.603).
ConclusionsIn-hospital mortality and readmission rates are similar in patients with AMI-SCAD and AMI–non-SCAD when adjusted for the differences in baseline characteristics. These findings underscore the need to optimize the management, treatment, and clinical follow-up of patients with SCAD.
Keywords
Spontaneous coronary artery dissection (SCAD) is a rare but increasingly recognized cause of acute myocardial infarction (AMI).1–8 It affects mainly middle-aged women and usually presents as acute coronary syndrome (ACS). Early coronary angiography in patients with ACS, intracoronary diagnostic techniques, and a high index of suspicion in the appropriate clinical contexts have improved diagnosis of this clinical entity.1–5 Until recently, the available evidence consisted of case reports and small retrospective series, but in recent years, larger, well-designed registries have been published, with systematic data collection and protocol-based clinical follow-up.9–13 Recently, scientific societies on both sides of the Atlantic have independently released 2 consensus documents.9,10 SCAD is often associated with noncoronary vascular disease (in particular fibromuscular dysplasia of large arteries) and some studies appear to support an associated genetic basis.9–13 Furthermore, unlike ACS of atherothrombotic etiology, in ACS caused by SCAD, conservative initial treatment is recommended, and coronary revascularization is reserved for cases with persistent or recurrent ischemia or complete vessel occlusion.9–13 However, there is still a lack of randomized studies to properly support clinical practice, from both a diagnostic and a therapeutic strategy perspective,14 due to the difficulty of organizing clinical trials given the low prevalence of the disease.
Some studies have used administrative databases as a source of information on SCAD.1–8,15 However, their specificity for identifying SCAD has been questioned,16 especially in light of the higher proportion of men observed in these studies, around 50%,2,16 compared with 12% in clinical registries.1,5 However, the use of administrative databases allows analysis of very large populations using well-established, widely-accepted, uniform criteria. This information is very important in diseases with a low incidence, such as SCAD.
This study aimed to determine the baseline characteristics, in-hospital mortality, and 30-day readmission rate in patients with AMI secondary to SCAD in Spain compared with other AMI patients, based on the minimum data set (MDS) of the Spanish National Health System (SNHS).
METHODSStudy populationThis was a retrospective observational study of events in patients admitted to SNHS hospitals with a diagnosis of AMI between 1 January 2016 and 31 December 2019, registered in the MDS and coded according to the International Classification of Diseases, 10th Revision-Clinical Modification (ICT-10-CM).17
The study population was divided into 2 groups: a) patients with a principal diagnosis of AMI (I21.x) without a secondary diagnosis of coronary artery dissection (I25.41) (AMI—non-SCAD), and b) patients with a principal or secondary diagnosis of coronary artery dissection and a principal or secondary diagnosis of AMI (AMI-SCAD). Multiple admissions resulting from interhospital transfers were considered a single event and attributed to the higher-level hospital. We used more restrictive exclusion criteria than those in previous studies on SCAD based on administrative databases,1–8 and excluded events in patients younger than 18 years, those registered as self-discharge, or with an unknown outcome at discharge, and those discharged to home after a stay of 1 day or less. To improve diagnostic accuracy, we excluded events with a diagnosis of accidental perforation or injury of the coronary artery during a medical procedure, atherosclerosis of a native or grafted coronary artery or of a transplanted heart, chronic ischemic heart disease, a secondary diagnosis of atherosclerosis, or a past medical history of AMI, coronary angioplasty, or aortocoronary revascularization surgery. Last, we excluded events not undergoing coronary angiography or coronary angioplasty. The ICD-10-CM codes and those of the clinical condition (CC) categories developed by Pope et al.,18 used to identify the exclusions, are shown in .
Due to the characteristics of the SNHS MDS and the anonymous nature of the data analyzed, there was no requirement for informed consent or ethics committee approval.
Statistical analysisFor the risk adjustment of in-hospital mortality and 30-day readmission rate, we used the Centers for Medicare and Medicaid Services (CMS) methodology for AMI,19,20 adapting it to the structure of the MDS, after grouping the secondary diagnoses included in the adjustment variables () according to the CCs, updated annually by the Agency for Healthcare Research and Quality.19,20 The multilevel logistic regression models were adjusted,21 including the patients’ demographic and clinical variables and a specific effect at the “hospital” level, and only the comorbidities with statistical significance and odds ratio (OR)> 1.0 were included. To estimate the adjustment models, we used backward stepwise elimination; the significance levels for selection and elimination of factors were P <.05 and P ≥ .10, respectively. Based on the specified models, we calculated the risk-standardized in-hospital mortality ratio (rIMR) and risk-standardized 30-day readmission ratio (rRAR) as the ratios between the predicted results (which consider the individual performance of the hospital where the patient was managed) and the expected results (which consider a standard performance based on the mean from all the hospitals) multiplied by the crude mortality rate or the crude readmission rate in the study population, such that, if the rIMR or the rRAR of a hospital is higher than the predicted crude rate, the probability of death or readmission in that hospital is considered higher than the mean of the hospitals studied.22
Calibration was analyzed graphically after grouping the patients into deciles with respect to the predicted probabilities and tabulating the predicted vs observed mean probabilities. Discrimination was assessed using the area under the receiver operating characteristic curve (AUROC)23.
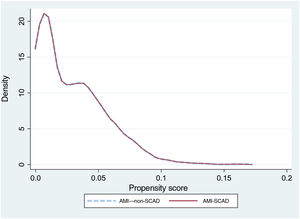
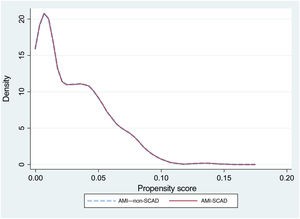
To reduce selection bias when comparing results, we assessed the impact of SCAD on the outcome variables using propensity score matching (option k-nearest neighbor matching, psmatch2, STATA), including the same variables used for the risk adjustment models. The matching was done in a 1:1 manner, without replacements. We calculated the probabilities of in-hospital death and readmission at 30 days, as well as the effect of the between-groups differences (average treatment effect) and the ORs with their 95% confidence intervals (95%CI). The graphical representation of the matching was performed using kernel density plots.
To study the possible effect of in-hospital mortality as a competing event for readmission, we estimated the subdistribution hazard function (Fine and Gray model, option stcrreg in STATA) and the result was compared with the estimate from a Cox proportional hazards model, in both cases taking the presence of SCAD as the independent variable.24
Quantitative variables are reported as mean±standard deviation and qualitative variables as frequency and percentage. The correlation between quantitative variables was analyzed with Pearson's r coefficient, and quantitative variables were compared using the Student t test or Wilcoxon or Mann-Whitney U test, as appropriate. Categorical variables were compared using the chi-square test or Fisher exact test. All comparisons performed were bilateral, and differences were considered significant when P <.05. Statistical analysis was performed using STATA 16 and SPSS v21.0.
RESULTSFor the study period, 182 685 events were identified from the MDS that met the inclusion criteria. A total of 181 660 (99.4%) were AMI–non-SCAD and 1025 (0.6%) were AMI-SCAD. After the exclusions, the study population was 120 231 (65.8%) and the group populations were 119 425 and 806, respectively (), with an incidence of 6.7 AMI-SCAD events per 1000 AMI.
The patient profile for both groups is shown in table 1. The AMI-SCAD group had a higher proportion of women (67.7% vs 31.3%; P < .001) and was younger (56.1±12.4 vs 67.2±14.4 years; P <.001). There were no significant differences between the 2 groups regarding the presence of ST-elevation AMI or cardiogenic shock at admission, but the AMI-SCAD group had a significantly lower prevalence of comorbidities than the AMI–non-SCAD group.
Differences in patient profile for AMI with or without spontaneous coronary artery dissection
| AMI–non-SCAD | AMI-SCAD | P | |
|---|---|---|---|
| Patients, n | 119 425 | 806 | |
| Age, y | 67.2±14.4 | 56.1±12.4 | <.001 |
| Women | 31.3 | 67.7 | <.001 |
| Fibromyalgia (M79.7) | 0.6 | 2.4 | <.001 |
| Smoker (Z72.0; F17.*) | 32.5 | 31.9 | .728 |
| Dyslipidemia (CC 25) | 43.5 | 35.4 | <.001 |
| Anterior myocardial infarction (principal diagnosis) | 23 | 26.3 | .029 |
| Nonanterior myocardial infarction | 37.1 | 33.6 | .044 |
| STEMI (I21. *, except I21.A1 and I21.4) | 59.5 | 60 | .810 |
| Unstable angina and other acute ischemic heart disease (I23.0, I23.1, I23.2, I23.3, I23.6, I23.7, I23.8, I24.1) | 1.5 | 2 | .24 |
| Metastatic cancer, acute leukemia, and other serious cancers (CC 8-9) | 1.2 | 0.7 | .255 |
| DM or complications of DM except proliferative retinopathy (CC 17-19, 123) | 27.9 | 11.5 | <.001 |
| Protein-calorie malnutrition (CC 21) | 0.4 | 0.2 | .493 |
| Chronic liver disease (CC 27-29) | 1.4 | 1.1 | .458 |
| Dementia or other specified brain disorders (CC 51-53) | 3.9 | 0.2 | <.001 |
| Major psychiatric disorders (CC 57-59) | 0.8 | 0.2 | .085 |
| Hemiplegia, paraplegia, paralysis, functional disability (CC 70-74, 103-104, 189) | 0.4 | 0 | .087 |
| Cardiogenic shock (R57.0) | 2.7 | 2.4 | .509 |
| Other cardiorespiratory failure or shock (CC 84 except 785.51) | 5.7 | 2.9 | .001 |
| Congestive heart failure (CC 85) | 16.5 | 7.2 | <.001 |
| Valvular and rheumatic heart disease (CC 91) | 14.5 | 9.2 | <.001 |
| Hypertension (CC 95) | 46 | 36.4 | <.001 |
| Stroke (CC 99-100) | 0.4 | 0.2 | .487 |
| Cerebrovascular disease (CC 101-102, 105) | 1.6 | 0.6 | .025 |
| Vascular disease and complications (CC 106-108) | 0.5 | 0.1 | .116 |
| Chronic obstructive pulmonary disease (CC 111) | 6.7 | 3 | <.001 |
| Pneumonia (CC 114-116) | 1.6 | 0.7 | .059 |
| Renal failure (CC 135-140) | 12.7 | 2.9 | <.001 |
| Trauma; other injury (CC 166-168, 170-174) | 1.2 | 1.7 | .143 |
AMI–non-SCAD, AMI of other etiology; CC, clinical categories18; DM, diabetes mellitus; PCI, percutaneous coronary intervention; SCAD, spontaneous coronary artery dissection; STEMI, acute ST-elevation myocardial infarction.
The data are expressed as mean ± standard deviation or percentage.
The crude in-hospital mortality rate was significantly lower in the AMI-SCAD group (3% vs 7.6%; P <.001). The risk adjustment model of in-hospital mortality () showed good discrimination (AUROC=0.87; 95%CI, 0.84-0.9) and calibration (). The rIMR was significantly higher in patients with SCAD (7.6±1.7% vs 7.4±1.7%; P=.019). Propensity score matching was performed for events in the AMI-SCAD group with the same number of events from the AMI–non-SCAD group. The patient profile for both groups after matching is shown in table 2 and figure 1. There were no significant differences in mortality between the 2 matched groups (OR, 1.15; 95%CI, 0.61-2.2; P=.653), such that the observed difference in in-hospital mortality in the crude analysis and with a different sign on adjustment, would likely have been small, if present, and of little clinical significance.
Patient profile after matching for in-hospital mortality
| AMI–non-SCAD (n=806) | AMI-SCAD (n=806) | SD | |
|---|---|---|---|
| Age, y | 55.9±12.5 | 56.1±12.4 | 0.013 |
| Women | 68.1 | 67.7 | 0.008 |
| STEMI (I21. *, except I21.A1 and I21.4) | 59.2 | 60 | 0.013 |
| Cardiogenic shock (R57.0) | 1.7 | 2.4 | 0.046 |
| Metastatic cancer, acute leukemia, and other serious cancers (CC 8-9) | 1 | 0.7 | –0.036 |
| Diabetes mellitus or complications of diabetes mellitus except proliferative retinopathy (CC 17-19, 123) | 11.8 | 11.5 | –0.009 |
| Chronic liver disease (CC 27-29) | 1 | 1.1 | 0.010 |
| Dementia or other specified brain disorders (CC 51-53) | 0.6 | 0.2 | –0.055 |
| Major psychiatric disorders (CC 57-59) | 0.2 | 0.2 | 0.000 |
| Hemiplegia, paraplegia, paralysis, functional disability (CC 70-74, 103-104, 189) | 0 | 0 | 0.000 |
| Other cardiorespiratory failure or shock (CC 84 except 785.51) | 3 | 2.9 | –0.006 |
| Congestive heart failure (CC 85) | 6.7 | 7.2 | 0.019 |
| Unstable angina and other acute ischemic heart disease (I23.0, I23.1, I23.2, I23.3, I23.6, I23.7, I23.8, I24.1) | 1.4 | 2 | 0.000 |
| Stroke (CC 99-100) | 0 | 0.2 | 0.043 |
| Vascular disease and complications (CC 106-108) | 0 | 0.1 | 0.055 |
| Pneumonia (CC 114-116) | 0.4 | 0.7 | 0.032 |
| Renal failure (CC 135-140) | 2.9 | 2.9 | 0.036 |
| Trauma; other injury (CC 166-168, 170-174) | 0.7 | 1.7 | 0.000 |
| Death | 2.6 | 3 | 0.077 |
AMI–non-SCAD, AMI of other etiology; CC, clinical categories18; SCAD, spontaneous coronary artery dissection; SD, standardized differences; STEMI, acute ST-elevation myocardial infarction.
The data are expressed as mean ± standard deviation or percentage.
The 30-day readmission rate for cardiovascular disease was similar in both groups (5% in AMI–non-SCAD vs 4.6% in the AMI-SCAD group; P=.67). The most common principal diagnoses leading to readmission in the AMI-SCAD group were AMI, angina, and other postinfarct complications (48.5% vs 28.1% in the AMI–non-SCAD group; P=.009). Readmission for congestive heart failure was more common in patients with AMI–non-SCAD (24.8% vs 9.1%; P=.037).
The rRAR was significantly lower in patients with SCAD (4.7±1% vs 4.8±1%; P=.015). The risk adjustment model for 30-day readmission rate showed acceptable discrimination (AUROC=0.69; 95%CI, 0.68-0.70) and calibration (table 3 and ). Propensity score matching was performed for 715 readmission events (88.7%) from the AMI-SCAD group and for the same number from the AMI–non-SCAD group (table 3 and figure 2). No significant differences were observed in readmissions between the 2 matched groups (OR, 1.14; 95%CI, 0.67-1.98; P=.603), such that, as occurred with the in-hospital mortality, the observed difference in 30-day readmission rate on the risk-adjusted analysis was, if present, not appreciable.
Patient profile after matching for 30-day readmission rate
| AMI–non-SCAD (n=715) | AMI-SCAD (n=715) | SD | |
|---|---|---|---|
| Age, y | 55.7±12.2 | 55.7±12.2 | 0.000 |
| Women | 61.8 | 61.8 | 0.012 |
| STEMI (I21. *, except I21.A1 and I21.4) | 25.7 | 25.7 | 0.006 |
| Serious infection; other infectious diseases (CC 1, 3, 7) | 1.1 | 1.1 | 0.053 |
| Metastatic cancer, acute leukemia, and other serious cancers (CC 8-9) | 0.1 | 0.1 | 0.048 |
| Diabetes mellitus or complications of diabetes mellitus except proliferative retinopathy (CC 17-19, 123) | 11.9 | 11.9 | –0.048 |
| Protein-calorie malnutrition (CC 21) | 0.1 | 0.1 | 0.037 |
| Iron deficiency or other/unspecified anemia or blood disease (CC 49) | 4.8 | 4.8 | 0.018 |
| Congestive heart failure (CC 85) | 5.9 | 5.9 | 0.012 |
| Rheumatic and valvular heart disease (CC 91) | 8.4 | 8.4 | 0.010 |
| Arrhythmias and other cardiac rhythm disorders (CC 96, 97) | 10.1 | 10.1 | 0.031 |
| Cerebrovascular disease (CC 101-102, 105) | 0.1 | 0.1 | 0.065 |
| Chronic obstructive pulmonary disease (CC 111) | 2 | 2 | 0.043 |
| Pneumonia (CC 114-116) | 0.6 | 0.6 | 0.000 |
| Renal failure (CC 135-140) | 3.5 | 3.5 | –0.064 |
| Trauma; other injury (CC 166-168, 170-174) | 0.3 | 0.3 | 0.048 |
| Readmission (%) | 4.1 | 4.6 | 0.000 |
AMI–non-SCAD, AMI of other etiology; CC, clinical categories18; SCAD, spontaneous coronary artery dissection; SD, standardized differences; STEMI, acute ST-elevation myocardial infarction.
The data are expressed as mean ± standard deviation or percentage.
The hazard ratios (HR) for the AMI-SCAD vs the AMI–non-SCAD (reference) group were similar when competing and proportional risks were analyzed: HR, 1.41 (95%CI, 1.0-2.98; P=.048) and HR, 1.45 (95%CI, 1.03-2.02), respectively, with a considerable difference in the median time to event (readmission): 18 vs 12 days. This indicates that readmission occurred earlier in the AMI-SCAD group, although the inclusion of competing risks did not change the result of the propensity score matching.
DISCUSSIONThe key findings from this study are: a) SCAD is a relatively rare but important cause of AMI in Spain; b) patients with AMI-SCAD are younger and more likely to be women and have a lower prevalence of comorbidities; c) our data question the widespread clinical perception that the outcome of AMI-SCAD is relatively benign; and d) our methodology allowed us to identify a SCAD population with a profile that is closer to that described in clinical studies than in studies based on administrative data1–8 (figure 3).
Central illustration. Study population and outcomes. AMI, acute myocardial infarction; ICD, international classification of diseases; MDS, minimum dataset; rRAR, risk-adjusted in-hospital mortality ratio; non-SCAD, no spontaneous coronary artery dissection; SCAD, spontaneous coronary artery dissection; SNHS, Spanish National Health System.
The in-hospital mortality outcomes were similar in patients with AMI-SCAD and AMI–non-SCAD. Although the crude rate was significantly lower in patients with AMI-SCAD than in those with AMI–non-SCAD (3% vs 7.6%; P <.001) and when we compared the risk-adjusted ratios, the sign of the difference changed, the effect had little statistical or clinical significance (7.6±1.7% vs 7.4±1.7%). After propensity score matching, the differences disappeared. There were also no statistically significant differences regarding the 30-day readmission rate for cardiovascular causes. The observed differences in the rRAR (4.7±1% vs 4.8±1%) have the same explanation as for rIMR. From a clinical perspective, the reasons for 30-day readmission differed between the 2 populations: in the patients with AMI-SCAD, ischemic causes were more common (48.5% vs 28.1%; P=.009) and in the AMI–non-SCAD group, heart failure was more common (24.8% vs 9.1%; P=.037). These data also confirm the need for very close clinical follow-up of all patients with AMI-SCAD.
Previous studies on spontaneous coronary artery dissection based on administrative dataStudies using administrative databases are very useful in highly-prevalent cardiological processes such as AMI or heart failure,25,26 but they are particularly appealing for providing additional or complementary information on less prevalent diseases, and they have been of particular interest in the analysis of SCAD. The results obtained so far from administrative-based studies in terms of in-hospital mortality and 30-day readmissions are not conclusive, and at times even discrepant.1–8,27 Most of the existing studies come from North America, such as the National Inpatient Sample (NIS) database, which has cost assessment as its main objective and has been used to analyze the characteristics and in-hospital mortality of these patients, or the Nationwide Readmission Database (NRD), used more for readmission analysis.
Krittanawong et al.2 analyzed NIS data (2004-2015) and found that, among 13 573 200 patients with ACS, 66 360 had a diagnosis (principal or secondary) of SCAD, with a mean age of 63 years, and only 44% women. Mortality in patients with ACS secondary to SCAD (4.2%) was not different from that in other patients with ACS and, among patients with SCAD, it was higher in women than in men (5% vs 3.5%; P < .001), although the analyses were not risk-adjusted. In another study, also using NIS data (2009-2014), Mahmoud et al.3 analyzed 752 352 women with AMI, of whom 7347 (0.98%) had a diagnosis (principal or secondary) of SCAD (mean age, 61.7 years). Mortality in patients with SCAD (6.8% vs 3.4%; P < .001) was higher than that in other patients with AMI. The women with AMI-SCAD were younger and had less comorbidity. The higher mortality in the patients with AMI-SCAD persisted after adjustment with logistic regression (OR, 2.4; 95%CI, 2.07-2.80) and propensity scoring (OR, 1.87; 95%CI, 1.65-2.11). The authors found that mortality in patients with SCAD decreased over the study period in parallel with a reduction in coronary revascularization as the initial treatment. Clare et al.1 analyzed the results from an administrative database for the state of California of patients with health insurance (2006-2016), to identify patients with SCAD. Of 26 598 patients with AMI, 208 (0.78%) had SCAD. Mortality at 1 month (1.4% vs 4.1%; P = .05) and 1 year (2.4% vs 8.8%; P <.001) was significantly lower in patients with SCAD. However, the patients with SCAD were younger and had fewer coronary risk factors and comorbidities than the other patients with AMI. When the propensity score was adjusted for these baseline differences (208 matched patients), the differences disappeared (1-year mortality, 2.4% with SCAD vs 1.4% without SCAD; P = .72). The investigators suggested that the lower mortality in patients with SCAD was simply due to their more favorable characteristics. Krittanawong et al.6 used the NRD database (2010-2016) to determine the characteristics of patients with “recurrence” of SCAD. Of 1836 patients with a diagnosis of SCAD (mean age, 56 years; 62% women), 495 had recurrence of SCAD during the first year. Being a woman was an independent predictor of recurrence. In-hospital mortality was 4% (3.4% after a first SCAD event and 5.8% for patients with recurrence; P = .17).
Few studies have used administrative databases from other geographical areas, which would allow determination of potential race-related variations in the disease. In Japan, Inohara et al.7 used a national administrative database to study SCAD in women with AMI from 2012 to 2017, finding 322 (0.5%) of 68 909 cases. The women with SCAD were younger, had fewer comorbidities, and were less likely to undergo revascularization than those with atherothrombotic AMI. In-hospital mortality was significantly lower in the women with SCAD (crude, 2.5% vs 7.6%; P=.001; age-adjusted, 2.5% vs 7.5%; P = .001; propensity score adjusted, 2.6% vs 6.5%; P = .033). Interestingly, in Japan, more than half (54%) of the women with SCAD underwent revascularization, in contrast to other geographical areas.
Administrative databases have also been used to study hospital readmissions in patients with SCAD compared with patients with ACS of atherothrombotic etiology. Gad et al.4 studied readmissions after AMI using NRD data (2010-2015). Of 2 654 087 patients with AMI, 1386 (0.052%) had SCAD. The patients with SCAD had a significantly higher 30-day readmission rate than the other AMI patients (12.3% vs 9.9%; P = .022). This result persisted after propensity score population adjustments. Most of the readmissions in patients with SCAD were for cardiac reasons, with new AMI being the most common reason (44.8%), followed by chest pain (20.1%). Readmission was more common in the first week after discharge and in patients who had undergonerevascularization. Virk et al.8 also used NRD data (2013-2014) to characterize readmissions in patients with SCAD at 90 days. Of 11 228 patients with ACS diagnosed with SCAD, 2424 (21.6%) were readmitted in the first 3 months, most during the first month. The most common reasons for readmission were a new ACS (25%) and heart failure (11%). Both studies showed that patients with SCAD have a high readmission rate.
These discrepant results may be explained, at least partly, by the different criteria used to select patients with SCAD in these administrative databases (including the ICD codes) and the lack of adjustment or different adjustment methods. In our study, we meticulously refined the selection criteria for SCAD patients, to try to optimize the specificity of the diagnosis. The calibration and discrimination of the models obtained were highly satisfactory. In addition, we compared the 2 populations with propensity score models. None of the previous administrative SCAD studies described met all these requirements of methodological and statistical quality. Last, the results of previous studies, performed in different clinical contexts, with different health care models, and different diagnostic strategies and approaches to SCAD,1–8 may not be extrapolatable to the characteristics and outcomes of AMI secondary to SCAD in this country.
LimitationsThis study has some limitations. The main limitation is inherent to the use of administrative databases for the study of clinical diseases.1–8 In this study, there were numerous relevant baseline characteristics that we were not able to analyze, such as angiographic and anatomical data (culprit artery, specific classification of SCAD, left ventricular ejection fraction), medications given, and the precise characteristics of the revascularization procedures performed, which could have affected the results. All the studies on SCAD based on administrative data have a lower proportion of women than the clinical registries. Although, due to the selection method used in our study, the proportion of women was higher than in other administrative studies on SCAD,5,9–13 it was still lower than that in clinical registries. Another limitation is due to the use of population identification criteria that were particularly aimed at optimizing the specificity and comparability of the CCs analyzed, which meant a considerable number of events were excluded, something which may represent a potential selection bias.
CONCLUSIONSSCAD is an uncommon cause of AMI that primarily affects middle-aged women and, although its crude mortality rate, which was lower than that of the AMI–non-SCAD group, would appear to indicate a more “benign” disease, there were no clinically relevant differences in rates of in-hospital mortality and cardiovascular readmissions once these were risk-adjusted. New studies are needed to improve the diagnosis, clinical focus, and treatment of patients with this clinical entity.
FUNDINGThis study was funded thanks to an unconditional grant from Menarini (RECALCAR project).
AUTHORS’ CONTRIBUTIONSF. Alfonso: concept and design, data analysis and interpretation, and writing of the article. C. Fernández-Pérez: concept, design, and data analysis and interpretation. M. García-Márquez: data collection, analysis and interpretation. M. García-Guimaraes: critical review of intellectual content. J.L. Bernal: data interpretation and writing of the. T. Bastante: critical review of intellectual content. D. del Val: critical review of intellectual content. N. del Prado: data collection, analysis and interpretation. J. Elola: concept and design, data collection, analysis and interpretation, and writing of the article. All authors have reviewed and accepted the final version.
CONFLICTS OF INTERESTNone.
To the Department of Health for the help provided to develop the RECALCAR project, with special thanks to the Health Information Institute.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2022.04.017