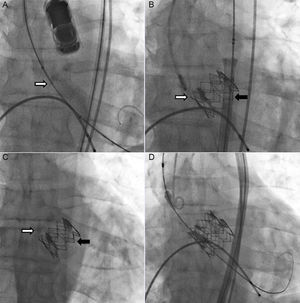
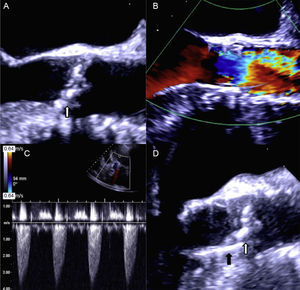
A 65-year-old man with severe degenerative aortic stenosis (Figures 1A and 2A) and preserved systolic function was evaluated by the Heart Team of our institution for surgical aortic valve replacement vs transcatheter aortic valve implantation (TAVI). A TAVI procedure was chosen because he had a heavily calcified aorta. He underwent a preprocedure computed tomography angiography to select the route, prosthesis type and prosthesis size for the procedure. He had no significant peripheral artery disease, and we therefore chose a transfemoral route. His aortic root area measured 430mm2 on computed tomography angiography. The aortic root diameter measured on 2-dimensional transesophageal echocardiography was 24mm. We therefore chose an Edwards Sapien XT 26mm valve. The TAVI procedure was undertaken (under transesophageal echocardiography) with balloon predilation, and no postdilation, without immediate complications. The balloon was prepared according to the manufacturer's recommendations (ie, no soft preparation, by using a lower amount of balloon saline, was undertaken). Fluoroscopy (Figure 1A and Figure 1B, and ), and transesophageal and transthoracic echocardiography (Figure 2A and Figure 2B, and ) confirmed appropriate prosthetic positioning with a mean gradient of 9mmHg and absence of relevant paravalvular leaks. However, 24hours after the procedure, a follow-up transthoracic echocardiogram was performed and a de novo mean aortic gradient of 42mmHg was revealed (Figure 2C). The prosthetic valve had migrated retrogradely into the outflow tract, thereby uncovering the native valve, which was functioning again (Figure 1C and Figure 2D, ). We reviewed the case and concluded that the prosthetic valve might have been positioned slightly too low and might have suboptimally expanded during the procedure. After discussion of the case in the Heart Team, a valve-in-valve procedure with a second 26-mm Edwards Sapien XT valve was chosen (Figure 1D and ). This enabled successful treatment of the aortic stenosis and prevented further migration of the original prosthesis. The patient was discharged 3 days after the second procedure and at 1 year of follow-up he is free of heart failure and the prosthesis is working adequately.
A, Echocardiographic images depicting preimplantation. B, Immediate postimplantation flow through prosthesis. C, Left ventricle-aorta gradient following prosthesis migration. D, Two-dimensional image of the prosthesis after migration. White arrow pointing toward native valve calcium. Black arrow pointing toward prosthesis.
Retrogade migration of the prosthetic valve following TAVI is rare. It can occur during the procedure,1 within the first few days after the procedure2 or subsequently.3,4 The first step in developing a solution is to identify the contributing factors for migration. These range from prosthesis malpositioning (ie, too low),1 suboptimal valve expansion, uneven or insufficient aortic annulus calcification leading to inadequate prosthesis fixation, aortic paravalvular regurgitation, valve undersizing,4 and anatomical or functional bicuspid valves. In our patient, the first cause was deemed responsible for this complication, although we cannot be sure that underexpansion did not also occur. It is as important to identify the true causes of migration as it is to exclude other factors. This enables appropriate solutions to be selected and also avoids potentially damaging ones. For example, wrongly considering valve undersizing as a cause of migration may lead to the subsequent use of an oversized valve with a high risk of further damage. Additionally, the use of fully repositionable valves may reduce the risk of malposition and migration. We also speculate that direct implantation (without predilation) could reduce the risk of this complication.
Once the causes of the migration have been determined, a surgical or percutaneous approach must be quickly chosen in a Heart Team setting, as the consequences of valve migration can be catastrophic if the valve extends beyond the outflow tract into the left ventricle cavity. In the few reports describing this complication, surgery was the preferred method in almost all published cases. Indeed, we found only 1 case in which this problem was solved by a percutaneous approach,5 but that was a case of valve undersizing and the complication still occurred during the procedure.
While surgery was considered in this case, we believed a valve-in-valve procedure was the safest way to solve the problem because the prosthesis was not interfering with the mitral apparatus, the patient had a porcelain aorta, and the migration was a consequence of valve subexpansion and slightly low positioning. The second prosthesis would be fixed not only in the native annulus but also on the original prosthesis, thereby preventing migration of both valves. This approach seems to have been justified by the patient's favorable outcome.