Vitamin K antagonists (VKAs), like direct oral anticoagulants (DOACs), reduce the risk of thromboembolic events in patients with atrial fibrillation (AF) in the absence of moderate to severe mitral stenosis or a prosthetic mechanical valve. VKAs can be challenging to prescribe due to their narrow therapeutic index. Time in therapeutic range (TTR), which is an estimate of the percentage of time a patient's international normalized ratio (INR) is within target values, is a measure of the quality of VKA treatment.1
DOAC prescription in Spain is strictly governed by the Ministry of Health's therapeutic positioning report, which provides guidance on the use of these drugs for preventing stroke and systemic embolic events in patients with nonvalvular AF.2 The current report stipulates that VKAs must be administered for at least 6 months before TTR can be calculated, and that INRs from the first month should not be included. The target INR range is 2.0 to 3.0, and patients with a TTR <65% according to the Rosendaal method or <60% according to the direct method can be considered for a switch to a DOAC.3
Based on clinical experience, we believe that an evaluation period of 6 months is excessive, not only because of the higher risk of iatrogenic adverse events during this time, but also because of the increased costs to the public health care system associated with longer periods of suboptimal anticoagulation.4 We therefore designed a study to determine whether INR control at 3 months could predict control at 6 months.
The data for our study were obtained from the CardioCHUVI-AF Registry at Complejo Hospitalario Universitario de Vigo in Vigo, Spain. We included 16 202 patients diagnosed with AF between January 2014 and January 2018. The following patients were excluded: AF patients with moderate to severe mitral stenosis or a prosthetic mechanical valve (n=350), nonanticoagulated patients (n=3885), patients on anticoagulation therapy with heparin (n=184) or a DOAC (n=1543), and patients on VKA treatment with a follow-up time of less than 6 months (n=165) or without INR test results over a period of 6 months (n=1108). The final cohort thus consisted of 8967 AF patients anticoagulated with a VKA. The study was approved by the local ethics committee, which deemed informed consent unnecessary due to the retrospective, anonymous nature of the study.
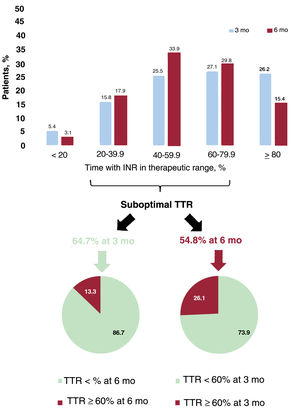
At 6 months, 54.8% of the patients (n=4916) had poor anticoagulation control (TTR <60%) and therefore met the criteria for switching to a DOAC. Of these, 3631 (73.9%) also had a suboptimal TTR at 3 months (figure 1).
On analyzing the data for 3 months, 46.7% of patients (n=4187) had a TTR <60%, and 86.7% of these (n=3631) still had suboptimal control at 6 months. Just 13.3% thus had INR values within the target range (TTR >60%) at 6 months (figure 1). Of the 4780 patients with good anticoagulation control at 3 months, 3495 (73.1%) continued to have INR values within the target range at 6 months. The remaining 1285 (26.9%) had poor control. Good anticoagulation control at 3 months therefore does not guarantee good control at 6 months.
In our study population, with a median follow-up of 5.0 years [interquartile range, 3.0-5.8 years], poor anticoagulation control at 3 months was associated with a higher likelihood of bleeding (hazard ratio [HR], 1.30; 95% confidence interval [95% CI], 1.13-1.49; P<.001) and a similar likelihood of ischemic stroke (HR, 1.12; 95% CI, 0.92-1.36; P=.256). Poor anticoagulation control at 6 months, in turn, was associated with a higher likelihood of bleeding (HR, 1.28; 95% CI, 1.12-1.48; P<.001) and ischemic stroke (HR, 1.26; 95% CI, 1.04-1.53; P=.020). Just 0.2% of the population had an ischemic stroke within the first 6 months (0.1% in the first 3 months and 0.1% in the second 3 months), compared with 4.4% after that period. Similar findings were observed for bleeding. Just 0.6% of the population experienced bleeding in the first 6 months (0.4% in the first half of this period and 0.2% in the second), while 8.4% experienced bleeding afterwards.
Based on current evidence, there is no doubt that DOACs outperform VKAs for stroke prevention. In addition, they are associated with significantly fewer intracranial hemorrhages. It has also been shown that patients on DOACs with a suboptimal TTR (<66%) experience a greater relative reduction in major bleeding than those who spend more time in the therapeutic range.5 Accordingly, in the absence of contraindications, current clinical practice guidelines (CPGs) recommend DOACs as the treatment of choice for AF patients without mitral stenosis or a prosthetic mechanical valve. Prescribing rates for DOACs in Spain are still low, and many of the country's autonomous communities continue to favor VKAs, partly because of the guidance in the Ministry of Health's therapeutic positioning report.
Based on the findings of our study, it could be concluded that approximately 9 of every 10 patients with AF on VKA treatment will have suboptimal anticoagulation control at both 3 months and 6 months. Perhaps it is time to propose a change to the current guidance on DOAC prescription in Spain. Based on the available evidence, shortening the recommended time for considering a switch from a VKA to a DOAC would reduce the risk of major embolic and bleeding events.
In conclusion, and considering the data presented, the favorable pharmacokinetic and pharmacodynamic properties of DOACs compared with VKAs, the strength of CPG recommendations (1A), and recent results from clinical trials, meta-analyses, and observational studies, we believe that Spain's guidance on the use of DOACs for preventing stroke and systemic embolic events in patients with nonvalvular AF should allow, as currently recommended by CPGs, unrestricted prescribing of DOACs. Failing this, it should shorten the time during which patients are at high risk of major events from 6 months to 3 months.
FundingThis study received no funding.
Authors’ ContributionsAll the authors contributed to the study design and the writing and critical review of this manuscript.
Conflicts of InterestThere are no conflicts of interest.